导图社区 Neuroimaging in ASD
- 58
- 3
- 1
- 举报
Neuroimaging in ASD
Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in ASD: brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121-1134. doi:10.1016/S1474-4422(15)00050-2
编辑于2021-04-22 00:37:25- 脑科学
- 孤独症谱…
- Neuroimaging in ASD
Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in ASD: brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121-1134. doi:10.1016/S1474-4422(15)00050-2
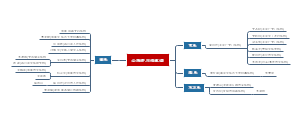
- 《自闭症谱系障碍者异常的大脑功能连接》 论文笔记
该图为张芬, 王穗苹, 杨娟华 撰写的《自闭症谱系障碍者异常的大脑功能连接》一文的思维导图。该论文主要研究ASD,并提出在未来的研究中,要综合分析脑功能与结构,关注年龄发展变化,并将任务状态、被试取样、数据分析标准等因素纳入考察。
Neuroimaging in ASD
社区模板帮助中心,点此进入>>
- Neuroimaging in ASD
Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in ASD: brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121-1134. doi:10.1016/S1474-4422(15)00050-2
- 《自闭症谱系障碍者异常的大脑功能连接》 论文笔记
该图为张芬, 王穗苹, 杨娟华 撰写的《自闭症谱系障碍者异常的大脑功能连接》一文的思维导图。该论文主要研究ASD,并提出在未来的研究中,要综合分析脑功能与结构,关注年龄发展变化,并将任务状态、被试取样、数据分析标准等因素纳入考察。
- 相似推荐
- 大纲
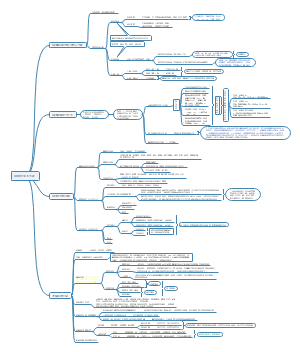
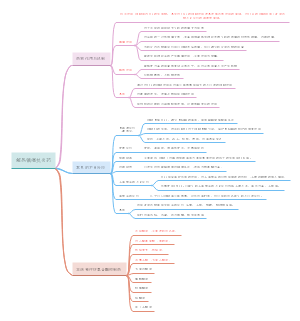
Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan
Atypical brain development
Early brain development
① investigation can only be reliably interpreted in the context of the wide neuroanatomical diversity within the general population ② both neural and non-neural tissue development should be considered to provide a more comprehensive assessment of atypical brain development in ASD.
toddlers with ASD (age 2–4 years) have, on average, a larger brain volume than typically developing children
this increased brain volume seems to disappear around the age of 6–8 years, when growth curves intersect
the neurodevelopmental trajectory of brain maturation is atypical in ASD and includes a period of early overgrowth followed by arrested growth and possibly a decrease in brain volume at older ages
the altered neurodevelopmental trajectory of the brain in ASD seems to vary across different brain regions, with the frontal and temporal lobes being affected more than the parietal and occipital lobes
This finding suggests that the temporal and regional sequence of typical early brain development is perturbed in ASD.
others have also reported generalised enlargements throughout the cerebral cortex in children with ASD between age 18 months and 35 months
not only affect the structure of isolated brain regions, but also lead to differences in brain anatomy and connectivity on a systems level.
ASD have an early enlargement of the brain that is already visible at around age 2 years.
Hazlett and colleagues did not identify an increase in cortical thickness
The finding of early enlargement of the brain in ASD, caused by an accelerated expansion of cortical surface area but not cortical thickness
radial unit hypothesis
cortical thickness is determined by the neuronal output from each radial unit amplified by intermediate progenitor cells, therefore, is related to the number of neurons produced in each unit.
cortical surface area has been associated mainly with radial unit progenitor cells, which divide at the apical ventricular surface.
Neural systems underlying autistic symptoms and traits
The components of the neural systems underlie ASD includes the frontotemporal and frontoparietal regions, amygdala–hippocampal complex, cerebellum, basal ganglia, and anterior and posterior cingulate regions.
Broca's area and Wernicke's area have been associated with social communication and language deficits
frontotemporal regions and the amygdala have been related to abnormalities in socio-emotional processing
the orbitofrontal cortex and the caudate nucleus (ie, frontostriatal system) might mediate repetitive and stereotyped behaviours
although abnormalities in these brain regions seem to be common in ASD, evidence suggests that the differences in these regions are not specific to the disorder.
Neurodevelopment across the human lifespan
brain development after early adolescence seems to be dominated by an accelerated age-related decline in total brain volume and its cortical thickness and surface area.
surface area decline more rapidly with age in individuals with ASD than in those without ASD both at the global and the local levels.
the rate of cortical development is not linear across the human lifespan, and precocious or delayed maturation in a group of individuals could lead to a substantial positive difference at one age and a substantial negative difference at another age
① large normative datasets characterising the developmental timecourse of different morphometric features in different brain regions will be needed to fully characterise the cortical ontogeny of ASD ② investigating volumetric features of brain anatomy, examination of differences in geometric features (eg, cortical folding) in ASD will also be important
Atypical cortical gyrification
The brain in ASD would be expected to show an increase in cortical folding
axonal white matter fibres might exert a pulling force on the overlying neocortex, and so might modulate cortical folding extrinsically via mechanical tension
Alternatively, cortical folding might be mediated by developmental changes intrinsic to the cortical sheet
linked to an accelerated expansion of the outer cortical layers relative to the deeper layers
linked to the microstructural complexity of the associated grey matter determined by dendritic arborisation, synaptogenesis, and the alignment of neurons in space
ASD has a significantly higher incidence of cortical malformations with compared with healthy controls
including polymicrogyria (too many small folds, thought to arise from atypical prenatal brain maturation), schizencephaly(clefts lined within the cortical grey matter), macrogyria (increased size of gyri), some sulci (eg, the Sylvian fissure) seem to be further along the principal axes of the brain
The local gyrification index at a given point on the cortical surface represents the amount of cortex buried within the sulcal folds and is computed as the ratio between the surface of a circular patch on the outer and the surface of the corresponding patch on the pial surface
In males with ASD aged 12–23 years, the local gyrification index is increased in bilateral posterior brain regions
The local gyrification index is significantly reduced in the left supramarginal gyrus in males aged 8–40 years
The local gyrification index is significantly reduced in the right inferior frontal and medial parieto-occipital cortices in children with ASD
particular pattern of cortical gyrification is variable across individuals
Except genetic and molecular processes, environmental factors and experience-dependent mechanisms also probably modulate cortical morphometry, and perhaps especially cortical folding, in contrast to conventional measurements of brain anatomy (eg, total or regional brain volume).
to elucidate the contribution of genetic and non-genetic factors to brain development in ASD, various cortical features should be examined to account for the large amount of phenotypic inter-individual variability typically noted in the brain in ASD.
Neural activation and functional connectivity
Functional networks in affected brain regions
Early functional MRI (fMRI) investigations focused exclusively on region specific differences in the magnitude of activation.
early studies examining neural responses to emotional faces initially showed reduced activation in face-specific perceptual regions in addition to limbic areas, particularly the amygdala
further examination showed that activation differences were strongly affected by experimental factors such as gaze direction and face familiarity
Because fMRI studies use different activation paradigms and task parameters, often in cohorts with diff erent ages and levels of severity, results vary markedly.
findings from many studies have shown decreased activation
in the social brain network during tasks related to emotional processing or social cognition, including the amygdala, temporal–parietal junction, insula, and inferior frontal cortex
in frontostriatal circuitry in response to cognitive control tasks and repetitive behaviours
in language circuitry during communication tasks
in reward circuitry
By contrast, abnormal increases in activation are found in response to irritants and direct gaze
these findings strongly support a link between regional structural brain abnormalities and functional sequelae, including both regional brain activation and phenotypic presentation.
In other studies, neural activity has been examined at the network level, focusing on brain connectivity either during task performance or in the resting state
In most fMRI studies in people with autism, functional connectivity during task performanc is decreased.
included only high-functioning older participants
By contrast, resting-state fMRI examines spontaneous fluctuations in brain activity, measuring differences in the correlation between regions in well defined functional networks
These studies remains substantial controversy regarding the nature of connectivity impairment in autism with researchers arguing in favour of under connectivity, over-connectivity, or unique patterns of both underconnectivity and over-connectivity depending on the brain region.
Furthermore, findings from a 2013 review suggested that connectivity results are affected by the age of the population studied
the nature and direction of connectivity differences in ASD might change across the lifespan.
Applications of graph theoretical approaches in ASD
Graph theoretical approaches describe properties of network dynamics and include metrics such as (1) modularity, which shows the extent to which clusters of regions are associated with one another and are segregated from other modules; (2) local efficiency, which describes how efficiently local regions can communicate together; and (3) global efficiency, which refers to how densely regions across the brain are connected to one another.
Network analysis during resting-state fMRI in ASD has yielded controversial results mainly because some crucial metrics are sensitive to motion artifacts
Findings from most studies have continued to support the broad notion that ASD have poorer connectivity in regions spanning long distances in the brain, whereas connectivity seems to be increased in local circuits
the development of domain-specific function modules is reduced. Although local connectivity within central hubs or rich clubsseems to be increased, the hub organisation is altered across the brain.
In children with autism, widespread patterns of developmental disconnection affect information processing at both the local and global levels; furthermore, the specific patterns of connectivity abnormalities relate to the severity of the autism phenotype.
Genetic risk factors for ASD and their association with measures of brain activation and connectivity
In general, comparisons of syndromic autism versus controls yield similar results to those of ASD versus controls with regard to reduced connectivity, brain overgrowth, and abnormal functional activation.
A few consistent differences, particularly enlarged caudate volumes and caudate connectivity, seem to be specific to these syndromes.
In several studies, associations have been reported between autism risk genes and brain connectivity
the gene CNTNAP2, which confers risk for selective language impairment and for the language phenotype in autism, is associated with abnormal structural and functional connectivity,and specifically a pattern of increased short-range and decreased longrange connectivity.
Variations in oxytocin receptor genes, which confer a risk for autism, have been associated with differences in both amygdala volume and functional connectivity of the hypothalamus.
the MET promoter variant—another autism risk gene—is related to both increased functional activation and decreased functional connectivity in neural networks associated with the processing of facial affect.
studies that have examined the relation between genes associated with autism and brain structure and function support a model of abnormal developmental connectivity that leads to both reduced functional activation and decreased development of functional connections, particularly in long-range pathways
Recent use of network analysis of gene expression profiles in tissue from patients with autism taken at post mortem suggest the potential to better link genes and brain development in autism. Because network analysis is independent of modality, these techniques offer the potential to combine functional and structural imaging results, providing a comprehensive and potentially integrative model linking anatomical abnormalities to functional and phenotypic outcomes.
The early proliferation of radial unit progenitor cells leads to an increase in the number of proliferation units, which in turn increases the number of ontogenetic columns, resulting in increased surface area.