导图社区 water related
- 27
- 0
- 0
- 举报
water related
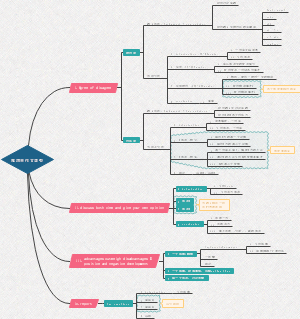
water related思维导图,包括:Dehydration和hydration两部分内容,喜欢的小伙伴可以点个赞哦!
编辑于2022-06-12 20:57:05- Dehydrati…
- water rela…
- alcohol
- hydrolysis
- hydration
- 相似推荐
- 大纲
water related
Dehydration
dehydration of primary amide
with Thionyl chloride (SOCl2)
Gnerate nitriles
mechanism. Socl2 nucleophylic attack, followed by E2 like
Imine and Enamine formation(Nu addition followed by dehydration)
Imine R2=NR
neucleophile is primary amines
ex. Schiff base in our body
can produced by Alanine react with PLP
a common way of its metabolism
nitriles can added by grignard reagent to yield Imine ion, then it adds a water to yield a ketone, by a machanism that is exact reverse of immine formation
p626
enamine R2N-CR=CR2
neucleophile is secondary amines
dehydration of alcohol
products
C=CR2
by reactants
3' alcohol
trearment
H3O+
E1
C=C
by reactants
2' alcohol
3' alcohol
treatment
POCl3
Pyridine
E2
E1Cb in biological system
Followed zaister's rule: major product is more stable alkene, due to C+ rearrangement
dehydration after adol reaction
between β-hydroxyl group and α-hydrogen
generate conjugated enones or α,β-unsaturated products
they have sharing pi electrons system/ holistic pi MO
more stable than nonconjugated enones
just like conjugated diene
condition
basic
E1cB
through enolate ion
Acidic
E1/E2
through enol
temperature
a bit higher than idol formation
hard to isolate adol, usually the product of carbonyl condensation
Value
move the idol formation equilibrium to product
removal of water
even though the initial equilibrium is unfavored
Hemiacetal dehydration to oxonium ion(C=O:+-R)
acid protonates hemiacetal's hydroxyl group, it then leaves to gave oxonium ion
double bond of oxonium ion is then added by another alcohol
water deprotonate the added alcohol, regenerate H3O+
yield actetal
definately reversiable
usually E1,E2,E2like,E1cB: depends on substrate and nucleophiles you applied
often convert -OH a better leaving group
protonation by acid
or substitute it by another group
hydration
hydrolysis
all acyl derivatives exept carboxylic acid can undergo hydrolysis
anhydride hydrolysis: 2 acids
direct add H2O, with out catalyst, so does below
we usually use bases such as pyridine or NaOH to neutralie generated HCl
acid chloride hydrolysis: acids
hydrolysis(react with water)
direct add H2O, with out catalyst, so does below
we usually use bases such as pyridine or NaOH to neutralie generated HCl
ester Hydrolysis: acids
Hydrolysis base catalyst
in base called sponification
yildes alkoxide ion and carboxylic acid
but alkoxide ions immediately deprotonate carboxylic acid
so may treat with aqueous acid if you want carboxylic acids
isotope labbling prove the nucleophilic acyl substitution mechanism
hydrolysis with acid catalyst
Usual mechanism: reverse of ficher esterification
acids first activated for nucleophilic attack by protonation of acid's carbonyl oxygen
then alcohol attacks the carbonyl carbon
The scond internal proton transfer transfer -OH2+'s proton to -OR oxygen make it a better leaving group
Enzyme mechanism: 2 sequential neucleophilic attacks
1. transesterification to give a acyl enzyme
with tetrahedral intermediate
2. hydrolysis to give an acid and free enzyme
with tetrahedral intermediate
both step requires general acid catalysts to make leaving group leave
ester hydrolysis: acids +ammine
similar(basically same) with ester acid/base catalyzed hydrolysis
base catalyst is hard because it can't protonates -NH2 to make a better leaving group
reversible
equilibria shifts by base deprotonates acids product
acid catalyst also followed by internal proton transfer
reversible
equilibria shifts by catalyst protonates NH3 product
but both requires heating in aqueous acid/ aqueous base
Biological enzyme catalysts cleavage
identical with ester cleavage with acyl enzyme intermediate
Hydrolysis
all nucleophilic acyl substitution can catalyst by both acid/base
but base can't accelerate the leaving step
Nitriles to carboxylic acid
p 625
first hydrolysis to amide
through a imine anion intermediate
mechanism: nucleophylic addition
just like other hydration, can be catalyst by acid/base
second hydrolysis to carboxylic acids
mechanism: nucleophylic acyl substitution
but only produce carboxylate ion, for reason of NH3 formation, so need further acidification
ketone/aldehyde hydration(form germdiols)
Catalysis
Acid catalysis mechanism
protonation of Carbonyl oxygen makes the carbonyl carbon more electrophilic
Water deprotonates the protonated diol intermediate then regenerates acid catalyst
Base catalysis mechanism
hydroxide ion is more nucleophilic than water
water protonates alkoxide ion then regenerates hydroxide ion
highly reversible
form germdiols
usually nucleophylic addition: catalyzed by both acid/base
the reverse is dehydration
like actetal formation
alcohol mimics water with same mechanism
ester formation
hemiacetal-actetal formation