导图社区 Redox agents
- 30
- 0
- 0
- 举报
Redox agents
Redox agents思维导图,包括:Oxidation、Reduction、summary等内容。希望对你有所帮助。
编辑于2022-06-12 21:00:54- mechanism
- Oxidation
- Redox ag…
- 相似推荐
- 大纲
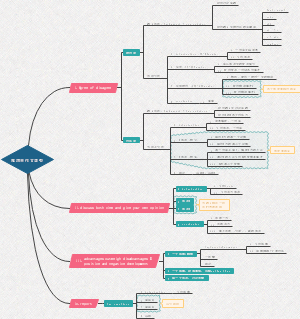
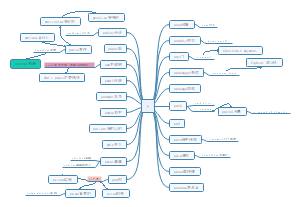
Redox agents
Oxidation
Substrate discrimination
primary alcohol
aldehyde
carboxylic acid
Secondary alcohol
ketone
Tertiary alcohol
no reaction
phenol
quinones
ketone
no reaction
Aldehyde
carboxylic acid
KMnO4
Oxidation of substituted alkyl benzene to benzoic acid
only for 1', 2' alkyl groups
replace alkyl group by carboxylic acid generate substituted benzoic acid
oxidize aldehyde into carboxylic acid
Hot HNO3
oxidize aldehyde into carboxylic acid
also can oxidlze alcohol into acid
Cr(VI) reagents
CrO3
usually in aqueous acid
Oxidation of a primary alcohol to a carboxylic acid
the aldehyde is a intermediate, but can't isolated
the oxidation is too quik
oxidize secondary alcohol to ketone
oxidize aldehyde to carboxylic acid
Na2Cr2O7
in aqueous acetic acid
inexpensive
for large scale oxidation
Oxidation of substituted alkyl benzene to benzoic acid
only for 1', 2' alkyl groups
replace alkyl group by carboxylic acid generate substituted benzoic acid
oxidize primary alcohol into aldehyde
oxidize seconary alcohol into ketone
Dess-Martin periodinane
usually in dichloromethane solvent
Non acidic, at lower room temperature
oxidize primary alcohol into aldehyde
oxidize seconary alcohol into ketone
Fremy's salt
(KSO3)2 NO
oxydize phenol/hydroquinones to quinones
this like ubiquinones in biological system
coenzyme Q
electron stransfer
Common mechanism
laboratory
alcohol
closely related to E2
first is the oxidant substites H of hydroxyl group
then generates a C=O by elimination of intermediate, leaving group is a reduced iodine (DMP) or metcal (Cr)
therefore require at least one H attach to the hydroxyl attached carbon
aldehyde
through intermediate 1,1 diol/ hyrates
formed by hydration of aldehyde
equilibrium amount
then hydrate is oxidized through the alcohol mechanism
therefore require at least one H attach to the carnonyl carbon
the 1,1 diol produced by ketone hydration do not have more H attached on the hydroxyl attached carbon..
phenol
radical
NAD+/NADP+
1.a base removes -OH proton
2. alkoxide ion transfers a hydride ion to coenzyme
ps: addition accors exclusively on the Re face of the NAD+ ring
adding a hydrogen with pro-R geometry
weak oxidants
Ag+
Cu2+
Br2
like industrial oxidize aldose into aldonic acid
Reduction
Mechanism
generally nucleophilic attack of H:-
nucleophilic addition
then protonation
for aldehydes/ketones
first nucleophilic acyl substitution
for acyl derivatives
to yield aldehyes/ketone intermediate/product
aldehydes often immediately oxidized to alcohol by nucleophilic addition
ex. LiAlH4
exept DIBAH
partial reduction
detailed different
section 14.6
chapter 16
16.6
16.8
13.3
LiAlH4
much more reactive than NaBH4
grayish powder
Only soluble in ether and tetrahydrofuran
react violently with water
dangerous
decompose and explode when heated above 120
reduction of aldehyde and ketones to alcohol
But can also reduce carboxylic acids and esters to alcohol
therefore LiAlH4 cover reduction of all carbonyl groups
unisolatable aldehyde intermediate
in ether solvent
should followed by protonation
Reduce nitriles to amines
Through amide intermediate
p 626
Reduce amide to amines
NaBH4
safety, easy handling
use in either water/alcohol solution
white, crystalline solid
can weighed in the open atmosphere
reduction of aldehyde and ketones to alcohol
reduce quinones to hyroqyinones
Reductive amination
aldehyde or ketone is treated with and amine in the presence of a reducing agent NaBH4
similar SnCl2
DIBAH
Diisobutylaluminum hydride
partially reduce an ester/thioester/acylphosphate to aldehyde
laboratory scale method
interestingly, it can't partially reduce carboxylic acids..
no one can partially reduce acids?
Yes, it's all or nothing
at -78℃ to avoid further reduction
in toluene solvent
NADH/NADPH
biological system..
can partially reduce thioester/acylphosphate to aldehyde
Grignard reagents
work as R:-
reduce ketone, aldehyde, acyl derivatives, even CO2---carboxylation produce carboxylate acids
can't stop at ketone/ aldehyde phase when reduce acyl derivatives, usually gives 2 identical substituted tertirary alcohol
Use R2CuLi can substitude only onece
But only effective for acid chloride!
also usful for conjugate addition of alkyl group
in polar aprotic solvent ether/THF
production
1°/2°/3° alkyl, alkenyl, aryl halide + Mg in ether or THF(polar aprotic solvent)
Ctalytic hydrogenation
H2 with pt catalyst
strong reduction forces reduce C=C(not aromatic), C=O(also nirtiles), and -NO2
double bonds are most reactive over catalytic hydrogenation
no aromatic
under normal strength, C=O in ester, aldehyde, ketone is untouched
less reactive
Mechanism
Syn stereochemistry addition
Lindar catalyst
reduce alkyne to alkene with syn stereochemistry
summary
oxidize
with out limit
KmnO4
HNO3
all steps
CrO3
one step(stop at aldehyde)
Dess-Martin periodinane
Na2Cr2O7
for large scale
special
fremy's salt
very weak oxidants only oxidize aldehyde: reducing sugar
Ag+, Cu2+
mechanism
probably must through enolateion intermediate
Br2
reduce
all steps
LiAlH4
dangerous
NAD(P)H
one step(stop at aldehyde)
DIBAH
NAD(P)H
but only work for some acyl dirivatives
ester
thioester
acylphosphate
only from second step(only from aldehyde/ketone)
NaBH4
easy to handle