导图社区 Chemical equilirbium
- 52
- 0
- 0
- 举报
Chemical equilirbium
Chemical equilibrium,内容有: Equilibrium Reversible and irreversible reaction Chemical equilibrium Chemical equilibirum constant,Kc Le Chatelier's principle Haber process RICE table.
编辑于2023-04-06 20:54:31 上海- 化学平衡
- 专业英语
- 应用化学
- Chemical equilirbium
- Chemical equilirbium
Chemical equilibrium,内容有: Equilibrium Reversible and irreversible reaction Chemical equilibrium Chemical equilibirum constant,Kc Le Chatelier's principle Haber process RICE table.
- 量子物理
量子物理(Quantum Physics),是研究物质世界微观粒子运动规律的物理学分支,主要研究原子、分子、凝聚态物质,以及原子核和基本粒子的结构、性质的基础理论,它与相对论一起构成现代物理学的理论基础。 量子物理包括两个部分:一是量子力学,它是原子层次的物理理论,是解释微观世界物质运动规律的理论;二是量子场论,它是研究场的基本规律,揭示了与物质本质相关的一些深刻问题
- 热力学基础
整理的热力学基础知识点总结汇总,复习必备。涵盖准静态过程 功 热量、内能 热力学第一定律、理想气体的等体过程和等压过程、理想气体的等温过程和绝热过程、循环过程 卡诺循环、热力学第二定律、熵增加原理和热力学第二定律的统计解释。
Chemical equilirbium
社区模板帮助中心,点此进入>>
- Chemical equilirbium
Chemical equilibrium,内容有: Equilibrium Reversible and irreversible reaction Chemical equilibrium Chemical equilibirum constant,Kc Le Chatelier's principle Haber process RICE table.
- 量子物理
量子物理(Quantum Physics),是研究物质世界微观粒子运动规律的物理学分支,主要研究原子、分子、凝聚态物质,以及原子核和基本粒子的结构、性质的基础理论,它与相对论一起构成现代物理学的理论基础。 量子物理包括两个部分:一是量子力学,它是原子层次的物理理论,是解释微观世界物质运动规律的理论;二是量子场论,它是研究场的基本规律,揭示了与物质本质相关的一些深刻问题
- 热力学基础
整理的热力学基础知识点总结汇总,复习必备。涵盖准静态过程 功 热量、内能 热力学第一定律、理想气体的等体过程和等压过程、理想气体的等温过程和绝热过程、循环过程 卡诺循环、热力学第二定律、熵增加原理和热力学第二定律的统计解释。
- 相似推荐
- 大纲
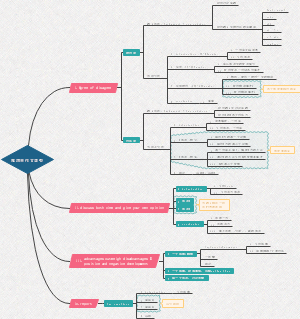
Chemical equilibrium
Equilibrium
the nature of equilibrium systems
A reaction is at equilibrium when the amounts of reactants or products no longer change.
the dynamic nature of a chemical equilibrium
Chemical equilibrium is a dynamic process, meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction.
Reversible and irreversible reaction
These equations typically have a unidirectional arrow (→) to represent irreversible reactions. Other chemical equations may have a bidirectional harpoons (⇌) that represent reversible reactions (not to be confused with the double arrows ↔).
irreversible reaction: reactions in which the reactants convert to products and where the products cannot convert back to the reactants.
reversible reaction: the reactants and products are never fully consumed; they are each constantly reacting and being produced
difference: In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction.
reversible reactions lead to equilibrium.If the reactants are formed at the same rate as the products, a dynamic equilibrium exists.
Chemical equilibrium
Chemical equilibrium is the state of a system in which the rate of the forward reaction is equal to the rate of the reverse reaction.
Reversible reaction. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved.
V(forward)=V(reverse).The rates of the forward and reverse reactions must be equal.
dynamic process. The forward and reverse reactions continue to occur even after equilibrium has been reached.
constant. At equilibrium, concentrations of all substances are constant.
change. A system that has reached equilibrium is changed, and the system will change accordingly to counteract the change.
Chemical equilibirum constant,Kc
The equilibrium constant (Keq) is the ratio of the mathematical product of the products of a reaction to the mathematical product of the concentrations of the reactants of the reaction. Each concentration is raised to the power of its coefficient in the balanced chemical equation.
The equilibrium constant (Keq) depends on the temperature of the reaction.For any reaction in which a Keq is given, the temperature should be specified.
The reaction quotient(Q),is used when questioning if we are at equilibrium.The calculation for Q is exactly the same as for K.
Le Chatelier's principle
Le Châtelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an equilibrium. If a chemical reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products or reactants, the equilibrium shifts in the opposite direction to offset the change.
Concentration Changes:if the system is changed in a way that increases the concentration of one of the reacting species , it must favor the reaction in which that species is consumed.
Pressure Changes:This only applies to reactions involving gases, although not necessarily all species in the reaction need to be in the gas phase.
if there are the same number of molecules on both sides of the equilibrium reaction, increasing the pressure has no effect on the position of the equilibrium.
Summary of Pressure Effects Adding products makes Qc greater than Kc . This creates a net change in the reverse direction, toward reactants. The opposite occurs when adding more reactants. Adding an inert gas into a gas-phase equilibrium at constant volume does not result in a shift. When the volume of a mixture is reduced, a net change occurs in the direction that produces fewer moles of gas. When volume is increased the change occurs in the direction that produces more moles of gas.
Temperature Changes: The main effect of temperature on equilibrium is in changing the value of the equilibrium constant.
Summary of Temperature Effects Increasing the temperature of a system in dynamic equilibrium favors the endothermic reaction. The system counteracts the change by absorbing the extra heat. Decreasing the temperature of a system in dynamic equilibrium favors the exothermic reaction. The system counteracts the change by producing more heat.
Catalysts: Adding a catalyst makes absolutely no difference to the position of equilibrium, and Le Châtelier's principle does not apply. A catalyst speeds up the rate at which a reaction reaches dynamic equilibrium.
Haber process
The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen
Composition: In some reactions you might choose to use an excess of one of the reactants. You would do this if it is particularly important to use up as much as possible of the other reactant. The mixture of nitrogen and hydrogen going into the reactor is1:3.
Temperature: You need to shift the position of the equilibrium (Equation 1) as far as possible to the right in order to produce the maximum possible amount of ammonia in the equilibrium mixture.Jowever, the lower the temperature you use, the slower the reaction becomes.400 - 450°C is a compromise temperature.(high proportion 15% and short time)
Pressure: The higher the pressure the better in terms of the rate of a gas reaction.However, Very high pressures are very expensive to produce on two counts. 200 atmospheres is a compromise pressure chosen on economic grounds.
Catalyst: The catalyst has no effect whatsoever on the position of the equilibrium. However, the catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reaction. Separating the ammonia, By mixing one part ammonia to nine parts air with the use of a catalyst, the ammonia will get oxidized to nitric acid.
RICE table
RICE table
R stands for Chemical reaction.
I stands for initial concentration. This row contains the initial concentrations of products and reactants.
C stands for the change in concentration. This is the concentration change required for the reaction to reach equilibrium. It is the difference between the equilibrium and initial rows. The concentrations in this row are, unlike the other rows, expressed with either an appropriate positive (+) or negative (-) sign and a variable; this is because this row represents an increase or decrease (or no change) in concentration.
E is for the concentration when the reaction is at equilibrium. This is the summation of the initial and change rows. Once this row is completed, its contents can be plugged into the equilibrium constant equation to solve for Kc.
Calculating Equilibrium Constant Value
Calculate equilibrium concentrations from the values of the initial amounts and the Keq. we presume that the reaction will proceed in the forward direction to make products. and let us assign it a variable. According to the balanced chemical equation, we know the equilibrium concentrations of our species. We can substitute these concentrations into the Keq expression for this reaction and combine it with the known value of Keq. We formalize this process by introducing the RICE chart, where RICE stands for reaction, initial, change, and equilibrium.