导图社区 Enzyme kinetics
- 22
- 0
- 0
- 举报
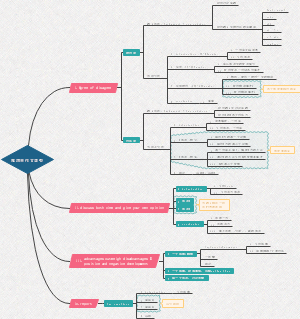
Enzyme kinetics
Enzyme kinetics思维导图,主要包括:steady state kinetic和spresteady state kinetics两部分内容。
编辑于2022-06-11 15:50:21- Enzyme ki…
- Vmax
- presteady…
- Vo
- 相似推荐
- 大纲
Enzyme kinetics
steady state kinetics
Vo
in responce to [S]
increases when substrate concentration increases
But not like normal catalyst: V=[S]k, catalyst only lower k, so increasing partern is linear
with [S] increase Vo usually increases slower and slower and finally stop increases (Vmax)
Vmax
what Vmax possibly means?
a realisitic modle comes up
and through reasoning we build up a kinetic equation
[S]+[E]↔[ES]→[E]+[P]
rate limiting step is [ES]→[E]+[P]
its k is kcat
this step is completely irreversible
means for this modle enzymes, the reverse reaction is not catalysted
It's true for most enzymes
key different from inorganic catalysts
accelerate both forward and reverse reaction
not change on Keq
so enzymes can further move the equilibrium?
immagine you drop some enzymes into a equilibriumed reaction system
in normal uncatalyst equilibrium, kforward*[S]=kreverse*[P]
if must further moved because at that instance k forward>>k forward before, but k reverse doesn't change, and [S] and [P] doesn't change
k forward increase, but k reverse increases under same degee
1 biggest doubt: how does the free energy changes overcomed?
ΔG=ΔGo+RTnQ
ΔGo and RT lnQ seems not changed during equilibrium situation?
yes, at least it can't explained from this equation
So the ΔGo couldn't be used, since it's measured not adding any those enzymes their
dynamic equilibrium through enzyme
it is assumed absolutely irreversible, but actually it's not true!, a "double reaction coordinate" from forward and reverse is not exist。。。
The reverse is neglected under our steady state kinetics model but do exist
neglect because the second step's ΔG is too negative
and it's do faster than normal(catalysted)
so equilibrium never changed by any catalyst (only lower activation energy)
first law of thermal dynamics: you can't give energy from nothing
answer another similar question
why we need kinase/ phosphatase for one process
Because they are not reverse reactions。。
phosphorylation most
substrate level
ATP+moleclue---P-molecule + ADP
very spontaneous
oxidative
ADP+pi----ATP
formula it self is crazy not spontaneous
spontaneous because mechanical energy invested
It's not chemically easily explainable
dephosphorylation most
P-molecule---pi+molecule
also spontaneous
so you know why.. they are not essentially reverse processes
equilibria is determined inextricably by standard free energy change
alternative: not dynamic equilibrium through enzyme, but static equilibrium, enzyme never turned because its own machenical equilibrium
what ever which one is true, the thermal dynamic laws can't be violated
infront of laws, impossible is impossible
like carrier proteins
binding energy
potential stabalization through very specific noncovalent molecular recognation
a very good alternative transition state
can be used to lower energy barrier/activation energy
The first step is irreversible
The real reaction's equilibrium is built on this step?
[ES]=[total E]
[E]=0
all enzymes are occupied
It's not realistic that all the inorganic catalyst is occupied
like protons: they are too small and too numorous
So what the equation can help us know sth?
Km= the [S] for V=1/2 Vmax
What can it predicts?
The biggest benifit is that if the catalyst modle is realistic, we can accurately estimate its Vmax, kcat(k2), and Km, with out really measured them, but by only solving the equation with several values measured on it
in realistics there are many deviations to accurately quantification, but at least the general partten is correct
The equation with Input [S]/output[Vo]
depicts their relation ship under realistic modle
presteady state kinetics
accelerates then reach steady Vo
steady only when excess [S] applied, the reaction do not significant changes [S]
Puzzels: not seen in normal catalysts
no measurable accelerates, but directly reach maximum speed.
if you ignore the time for diffusion
because enzyme proteins are activated by first several catalytic events?
induce fit?