导图社区 有机化学思维导图
- 135
- 1
- 0
- 举报
有机化学思维导图
耗时十几个小时为班上的同学们准备的有机化学期末考试复习资料。教材上都是以官能团这条“经线”展开,而这份知识点总结采取“纬线”的方式,从各个不同的角度把知识点串起来,便于它们的记忆和彼此联系。因为班上还有几位留学生,所以是双语的。我上网看了一下,所有的有机化学的思维导图都是按照官能团来做的,我的这个应该会比较有价值,所以分享给大家。有需要的同学和老师可以下载PDF版本的。当然可能有不完善的地方,欢迎帮我指出,一起把它做好。
编辑于2022-06-21 09:12:02- 经线
- 相似推荐
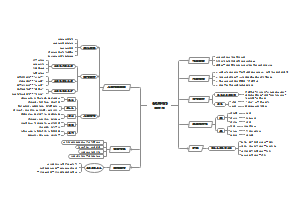
- 大纲
有机化学 思维导图 (xx.yy) xx为chapter yy为页码 PKU-MSE-2022
反应类型 reaction type
加成反应 addition
规则 rules
马尔科夫尼科夫规则 (氢多加氢)(7.19) Markovnikov's rule
背后的机制(mechanism): 生成取代基多的碳正离子 multi-substituted carbon cation
反马尔科夫尼科夫规则 anti-Markovnikov's rule
按底物分类 classification base on substrates
烯烃(7.16,8) alkene
X2卤素加成(反式加成)(8.3) halogen addition (trans-addition)
HX加成(马氏,反式)(7.16) HX addition (Markovnikov, trans-addition)
HOX次卤酸加成(马氏,反式)(8.6) HOX addition (Markovnikov, trans-addition)
H2O加成(马氏,反式)(8.9) H2O addition (Markovnikov, trans-addition)
以上反应可能有碳正离子重排问题 note: the above reactions may involve carbon cation rearrangement
硼氢化氧化(反马氏,顺式)(8.13) hydroboration-oxidation
催化氢化(顺式)(8.18) catalytic hydrogenation
炔烃 alkyne
与X2,HX加成(马氏,反式)(9.4) X2, HX addition (Markovnikov, trans-addition)
H2O加成(马氏,反式)(9.5) 注意产物 H2O addition (Markovnikov, trans-addition) pay attention to the product formed
硼氢化氧化(反马氏,顺式)(9.8) 注意产物 hydroboration-oxidation, pay attention to the product formed
苯炔(16.50) benzyne
醛和酮(19.8) aldehyde ketone
水作为亲核试剂(19.12) water as nucleophile
HCN作为亲核试剂(19.15) HCN as nucleophile
增碳反应 homologation
负氢和格氏试剂做亲核试剂(19.16) hydride and Grignard reagent as nucleophile
还原为醇 reduction to alcohol
胺作为亲核试剂(19.17) amine as nucleophile
亚胺,烯胺 imine, enamine
羟胺作为亲核试剂(19.18) hydroxylamine
肟 oxime
肼作为亲核试剂(19.21) hydrazine as nucleophile
腙 hydrazone
沃尔夫-凯惜纳-黄鸣龙还原反应 Wolff-Kishner-Huang reactioin
醇作为亲核试剂(19.25) alcohol hydrazine
(半)缩醛,(半)缩酮 (semi-)acetal, (semi-)ketal
膦叶立德作为亲核试剂(19.28) Wittig反应,1979诺贝尔奖 ylide hydrazine Wittig reaction, 1979 Nobel Prize
烯烃 alkene
腈 nitrile
与水加成(20.22) reaction with water
负氢加成(20.23) reduction by hydride
与格氏试剂反应(20.24) addition by Grignard reagents
消除反应 elimination
规则 rules
扎依切夫规则 (氢少去氢)(11.26) Zaitsev's rule
背后的机制(mechanism): 生成取代基多的烯烃 multi-substituted alkene
反扎依切夫规则 anti-Zaitsev's rule
一般是位阻原因,见霍夫曼消除 steric effect, refer to Hofmann elimination
机制 mechanism (11.28)
E1 (11.36)
形成碳正离子中间体 via carbon cation intermediate
E2 (11.30)
反式共平面 anti periplanar geometry
E1cb (11.38)
形成碳负离子中间体 via carbon anion intermediate
按底物分类 classification based on substrates
卤化物的消除(11.26) alkyl halide elimination
醇的消除(扎依切夫规则)(17.30) elimination of hydroxyl group
酸性条件(E1) acidic conditions
碱性条件(E2) basic conditions
季铵盐的消除——霍夫曼消除(E2, 反扎依切夫)(24.31) Hofmann elimination
取代反应 substitution
亲核取代 nucleophilic substitution
SN1(11.14)
底物,离去基团,亲核试剂和溶剂的影响 substrate, leaving group, nucleophile and solvent effects
SN2(11.6)
底物,离去基团,亲核试剂和溶剂的影响 substrate, leaving group, nucleophile and solvent effects
芳环的亲核取代反应(16.44) nucleophilic aromatic substitution
苯炔(16.50) benzyne
亲电取代 electrophilic substitution
主要指芳香环上的各种取代反应
芳香环的亲电卤化(16.4) electrophilic halogenation of aromatic compounds
溴化:Br2, FeBr3 bromination
氯化:Cl2, FeCl3 chlorination
氟化:F-TEDA-BF4 fluorination
碘化:I2, CuCl2 Iodination
芳香环的硝化(16.9) electrophilic nitration of aromatic compounds HNO3 + H2SO4
芳香环的磺化(16.10) electrophilic sulfonation of aromatic compounds SO3, H2SO4
芳香环的傅克烷基化和傅克酰基化(16.14) Friedel-Crafts alkylation and acylation
烷基化 alkylation
卤代烷,AlCl3(催化剂) alkyl halide, AlCl3(catalyst) 涉及碳正离子重排 may involve carbon cation rearrangement
酰基化 acylation
酰氯,AlCl3(当量) acyl chloride, AlCl3(1 equivalent) 没有碳正离子重排问题 no carbon cation rearrangemtn issue
取代基效应(16.20) substituent effect 诱导效应和共轭效应的叠加 inductive effect and conjugative effect
活化与钝化(16.20) activate or deactivate
连有钝化基团不能发生傅克反应 substrates with deactivation substituent do not undergo FC reaction
定位效应(16.21) orientation
活化基团:邻对位定位 activation group: ortho- and para-orientated
弱钝化基团(卤素):邻对位定位 less deactivation group(halo): ortho- and para-orientated
钝化基团:间位定位 deactivation group: meta-orientated
自由基取代 radical substitution
烷烃的自由基卤化(3.29) alkane radical halogenation 一般不用做实验室合成方法,选择性差 not useful in lab synthesis, poor selectivity
烯丙基溴化反应(10.10) allylic radical bromination
桑德迈尔反应(24.41) Sandmeyer reaction
卤化(24.41) halogenation
氰基化(24.41) cyazation
羟基化(24.42) hydroxylation
氢化(24.43) hydrogenation
重排反应 rearrangement
克莱森重排(18.11) Claisen rearrangement
霍夫曼重排(24.26) Hofmann rearrangement
柯提斯重排(24.26) Curtius rearrangement
聚合反应 polymerization
自由基聚合(8.34) radical polymerization
逐步聚合(21.45) step-growth polymerization 聚酯,聚酰胺(尼龙) polyester, polyamide (nylon)
规则与选择性 rule and selectivity
区域选择性及规则 regioselectivity
(反)马氏规则(7.19) (anti-)Markovnikov's rule
(反)扎依切夫规则 (anti-)Zaitsev's rule
立体选择性及规则 stereoselectivity
顺式加成/消除 cis addition/elimination
反式加成/消除 trans addition/elimination
不对称催化 asymetric catalysis
2001年诺贝尔化学奖 2001 Nobel Chemistry Prize
化学选择性 chemoselectivity
试剂对官能团有选择性地反应 selectively react with certain functional groups
例如:SnCl2还原硝基,醛基不受影响(24.15) SnCl2 selectively reduces nitrogroup, while aldehyde can survive
立体专一性(14.20) stereospecificity
立体化学不同的底物分别单一地得到各自对应的立体化学不同的产物 products inherit stereochemistry information from their corresponding substrates
芳香性与休克尔规则(15.9) aromaticity and Hückel rule
芳香性 aromaticity
闭合环状平面型的共轭多烯pi电子数为4n+2 molecule has planar, monocyclic system of conjugation and contains a total of 4n+2 pi electrons
反芳香性 anti-aromaticity
具有4n个π电子而又具近似平面结构的环状化合物 molecule is cyclic, planar, and conjugated, but has 4n pi electrons
非芳香性 non aromaticity
既不是芳香,也不是反芳香,类似开链烯烃 neither aromatic nor anti-aromatic
瓦尔登翻转(11.2) Walden cycle iinterconverting
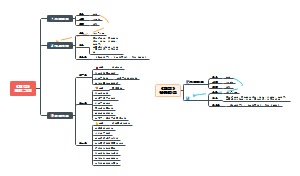
反应机理 reaction mechanism
极性反应(键的异裂)(6.5) polar reaction (heterolytic cleavage)
自由基反应(键的均裂)(6.5) radical reaction (homolytic cleavage)
周环反应(协同过程, 不区分异裂均裂)(18.11) pericyclic mechanism
Diels-Alder反应(14.17) Diesl-Alder reaction
克莱森重排(18.11) Claisen rearrangement
氧化反应 oxidation
烷烃的氧化 oxidation of alkanes
燃烧和卤化(3.29) combustion and halogenation
烯烃的氧化 oxidation of alkene
环氧化(8.23, 18.12) epoxidation
制备含氧三元环 preparation of oxygen- containing 3-membered ring
双羟化(8.24) di-hydroxylation
顺式二醇 cis-diol
OsO4氧化(8.25) OsO4 oxidation
反式二醇 trans-diol
环氧化水解开环 epoxidation and hydroxylative ring opening
制备羰基化合物(8.27) for carbonyl compound preparation
臭氧化(8.27) ozonization
高锰酸钾氧化(8.28) KMnO4 oxiation
高碘酸氧化(8.28) periodate oxidation
炔烃的氧化 oxidation of alkyne
高锰酸钾氧化得到羧酸(9.13) oxidation by KMnO4 will give carboxylic acid
芳环侧链的氧化(16.53) oxidation of aromatic compounds side chain
醇的氧化(17.34) oxidation of alcohol
Dess Martin氧化剂(17.35) Dess Martin oxidant
氧化一级醇到醛,二级醇到酮 oxidize primary alcohol to aldhyde, secondary alcohol to ketone
铬氧化剂(17.36) Cr oxidant
氧化一级醇到酸,二级醇到酮 oxidize primary alcohol to carboxylic acid, secondary alcohol to ketone
醛的氧化(19.7) oxidation of aldehyde
单糖的氧化(25.30) oxidation of monosaccharides
溴水氧化,只氧化醛基(25.30) aqueous Br2
稀释的硝酸氧化, 氧化醛基和一级醇, 得到二酸(25.33) dilute HNO3
还原反应 reduction
烯烃的还原 reduction of alkene
催化氢化(顺式)(8.18) catalytic hydrogenation
炔烃的还原 reduction of alkyne
催化氢化(顺式)(9.10) catalytic hydrogenation
过渡金属Pd,Pt,Rh等,加氢直接还原到烷烃 with Pd, Pt, or Rh transition-metal catalysts alkane will be obtained
Lindlar催化剂:Pd/CaCO3, Pb(OAc)2, quinoliine (9.10)
顺式加氢到烯烃 cis-alkene obtained
Li/NH3还原(9.12) Li/NH3 reduction
反式加氢到烯烃 trans-alkene obtained
芳环的还原(16.58) reduction of arene
酯的还原(21.36) reduction of ester
LiAlH4还原得醇 reduction by LiAlH4 affords alcohol
DIBAH还原得醛(20.37) reduction by DIBAH affords aldehyde
酰胺的还原(21.42) reduction of amide
单糖的还原(25.27) reduction of monosaccharides
卤化反应 halogenation
烷烃的卤化(3.29) halogenation of alkane
自由基取代反应 radical substitution
烯烃的卤化 halogenation of alkene
X2卤素加成(反式加成)(8.3) halogen addition (trans-addition)
HX加成(马氏,反式)(7.16) HX addition (Markovnikov, trans-addition)
HOX次卤酸加成(马氏,反式)(8.6) HOX addition (Markovnikov, trans-addition)
炔烃的卤化 halogenation of alkyne
与X2,HX加成(马氏,反式)(9.4) X2, HX addition (Markovnikov, trans-addition)
芳香环的亲电卤化(16.4) electrophilic halogenation of aromatic compounds
溴化:Br2, FeBr3 bromination
氯化:Cl2, FeCl3 chlorination
氟化:F-TEDA-BF4 fluorination
碘化:I2, CuCl2 Iodination
烯丙基溴化反应(10.10) allylic radical bromination
芳环苄位的卤化(16.54) halogenation of benzylic position
羰基alpha位的卤化 alpha halogenation of carbonyl compounds
醛酮的alpha位卤化(22.6) alpha halogenation of aldehyde and ketone
酸性条件(22.6) under acidic conditions
碱性条件(22.18) under basic conditions 卤仿反应 haloform reaction
羧酸的alpha位卤化(22.9) alpha halogenation of carboxylic acid
杂环的卤化(24.53) halogenation of heterocyclic compounds
胺化反应 amination
腈,硝基化合物还原(24.14) reduction of nitrile and nitro compounds
烷基卤化物的SN2反应(24.17) SN2 reactions of alkyl halides
一级胺的制备(24.19) preparation of primary amines
叠氮的还原(24.19) reduction of azide
盖布瑞尔反应(24.20) Gabriel reaction
醛酮的还原胺化(24.22) reductive amination of aldehydes and ketones
酮酸的还原胺化——制备氨基酸(26.14) reductive amination of alpha-keto acids
构建碳碳键的反应 carbon-carbon bond forming reaction
格氏试剂作为亲核试剂 Grignard reagents as nucleophiles
格氏试剂的生成(10.18) generation of Grignard reagents
与醛酮反应(17.20, 19.16) reaction with aldehyde and ketone
与二氧化碳反应(20.15) reaction with CO2
对腈加成(20.24) reaction with nitrile
与酯反应(21.38) reaction with ester
与环氧化合物反应(18.16-17) reaction with epoxide 注意区域选择性,从位阻小的一边进攻 attack to less hindered side
有机铜锂试剂作为亲核试剂 Gilman reagents, lithium diorganocopper compounds
与卤化物反应(10.21) reaction with alkyl halide
与酰氯反应得到酮(19.5) reaction with acyl chloride to afford ketone product
端炔去质子得到碳负离子作为亲核试剂(9.14) deprotonation of terminal alkyne forms carbon anion as nucleophiles
芳香环的傅克烷基化和傅克酰基化(16.14) Friedel-Crafts alkylation and acylation
羰基alpha位与亲电试剂的反应(22.5, 22.19) reaction of carbonyl compounds with electrophilies
丙二酸酯合成应用(22.20) the malonic ester synthesis application
乙酰乙酸酯合成应用(22.25) the acetoacetic ester synthesis application
酮的alpha位烷基化(22.31) alpha alkylation of ketone
腈的alpha位烷基化(22.32) alpha alkylation of nitrile
羰基化合物的缩合反应(23) carbonyl compounds condensation reaction
羟醛缩合(23.2) aldol condensation
自身羟醛缩合(23.3) self aldol condensation
有alpha氢的醛可以反应,酮一般很难 aldehyde with alpha-H undergoes this reaction, while ketone does not
混合羟醛缩合(23.13) mixed aldol condensation
避免出现多种产物的方法(23.14) avoid many products
分子内羟醛缩合成环(23.18) intramolecular aldol condensation
克莱森缩合(23.20) Claisen condensation
自身克莱森缩合(23.20) self Claisen condensation
混合克莱森缩合(23.22) mixed Claisen condensation
分子内克莱森缩合——狄克曼环化(23.25) intramolecular Claisen condensation: Dieckmann annulation
迈克尔共轭加成(23.28) Michael conjugated addition
烯胺参与的缩合反应——Stork烯胺策略(23.33) enamines undergo condensation: Stork enamin strategy
鲁滨孙环化(23.38) Robinson annulation
成环的反应 annulation reaction
全碳环 all carbon ring
烯烃的环丙烷化(8.30) cyclopropanation
Diels-Alder反应(14.17) Diels-Alder reaction
鲁滨孙环化(23.38) Robinson annulation
丙二酸酯合成法(22.23) the malonic ester synthesis
分子内羟醛缩合成环(23.18) intramolecular aldol condensation
分子内克莱森缩合——狄克曼环化(23.25) intramolecular Claisen condensation: Dieckmann annulation
杂环 heterocyclic
烯烃的环氧化(8.23) epoxidation of alkene
官能团的保护与去保护 functional group protection and deprotection
醇 alcohol
保护(17.40) protection
去保护(17.41) deprotection
醛和酮 aldehyde ketone
保护(19.25) protection
去保护(19.26) deprotection
羧酸 carboxylic acid
保护与去保护(26.25) protection and deprotection
胺 amine
保护(26.26) protection
去保护(26.27) deprotection
三氟乙酸 CF3COOH trifluro acetic acid
诺贝尔化学奖 Nobel Chemistry Prize
1912 格氏试剂 Grignard reagents
1950 Diels-Alder反应(14.17) Diels-Alder reaction
1963 Ziegler-Natta催化剂催化聚合反应 discovery of chemistry and technology of high polymers
1979 Wittig反应(19.29) Wittig reaction
1987 主客体化学(18.19) host-guest chemistry
2001 不对称催化 asymetric catalysis
2002 生物大分子的质谱分析法(12.12) mass spectrometry analysis of biomacromolecules
2005 烯烃复分解反应 olefin metathesis reaction
2010 钯催化的偶联反应(10.23) Pd-catalyzed cross-coupling reaction
2021 有机小分子催化 organocatalysis
人物 personage
墨子(前468-前376) Mo Zi 中国
最早具有原子朴素概念的科学家(1.2) 《墨子》:“非半不则不动,说在端”,“端,是无间也”
贝采里乌斯(1779-1848) J. J. Berzelius 瑞典
最早提出有机化学概念(0.9) organic chemistry concept
维勒(1800-1882) F. Wohler 德国
合成尿素,打破了有机化合物的“生命力”学说(0.10) synthesize urea
汤姆森(1856-1940) J. J. Thomson 1906 Nobel Physics Prize
汤姆森原子模型(1.3) Thomson's model
卢瑟福(1871-1937) E. Rutherford 新西兰/英国 1908 Nobel Chemistry Prize
卢瑟福原子模型(1.4) Rutherford's model
薛定谔(1887-1961) E. Schrödinger 奥地利 1933 Nobel Physics Prize
薛定谔方程(量子力学基本假设之一)(1.6) Schrödinger wave equation
巴斯德(1822-1895) L. Pasteur 法国
法国微生物学家、化学家,近代微生物学的奠基人 最美科学实验——拆分酒石酸(5.14) separation of tartrate enantiomers
格林尼亚(1871-1935) F. Grignard 法国 1912 Nobel Chemistry Prize
格氏试剂 Grignard reagent
桑格(1918-2013) F. Sanger 英国 1958 and 1980 Nobel Chemistry Prizes
测定胰岛素的氨基酸序列 determination of the amino acid sequence of insulin 测定DNA序列 determination of DNA sequence
伍德沃德(1917-1979) R. B. Woodward 美国 1965 Nobel Chemistry Prize
现代有机合成之父 the father of morden organic synthesis
科里(1928- ) E. J. Corey 美国 1990 Nobel Chemistry Prize
逆合成分析 retro-synthetic analysis
结构鉴定 characterization
质谱(12.4) mass spectrometry
确定分子量 molecular size and formula
测量质核比 measure m/z
红外(12.15) infrared spectroscopy
确定官能团 functional group
分子骨架振动吸收红外波段的光 molecule stretching or bending absorb IR
紫外(14.35) ultraviolet spectroscopy
共轭结构 conjugated pi electron system
紫外光激发分子内部电子能级的跃迁 electrons transit to higher energy level when absorb UV light
核磁(13) resonance spectroscopy
碳氢骨架 carbon-hydrogen framework
处于不同化学环境的核具有不通的自旋共振频率 Nuclei in different chemical environments have different spin resonance frequencies
热力学与动力学 thermodynamics and kinetics
化学反应热力学(6.22) thermodynamics
键的离解能(6.26) bond dissociation energy
生成焓 enthalpy of formation
吉布斯自由能 Gibbs free energy
化学反应动力学(6.29) kinetics
过渡态理论(6.29) transition-state theory
反应中间体(6.10) intermediates
活化能 activation energy
立体化学 stereochemistry
异构体类型分类(5.30) isomer classification
构造异构 constitutional isomers
立体异构 stereoisomers
对映异构 enantiomers (镜面对称) (mirror-image)
非对映异构体 diastereomers (非镜面对称) (non-mirror-image)
构形异构 configurational diastereomers
顺反异构 cis-trans diastereomers
烯烃的顺反异构 E/Z构型(7.8) alkene cis- and trans- isomers
环烷烃取代基的顺反异构(4.9) cycloalkane cis- and trans- isomers
对映异构体(5.3) enantiomers
手性碳原子 chiral carbon
RS构型判断(5.16) RS configuration
旋光度 specific rotation
重要概念 some concepts
外消旋体(5.28) racemate
一对对映异构体 a pair of enantiomers
内消旋体(5.25) mesomer
具有手性碳,但分子存在对称元素,无光学活性 possess chiral carbons and symmetry elements, non optically active
分子的构像 conformation
乙烷的构像(3.30) ethane's conformation
交叉 staggered
重叠 eclipsed
环己烷的构像(4.18) cyclohexane's conformation
椅式构象 chair conformation
平伏键(4.19) equatorial
直立键(4.19) axial
扭船式 twist-boat conformation
船式构像 boat conformation
价键与结构 bonding and structure
原子结构(1.3) atom description
汤姆森模型(1.3) Thomson's model
卢瑟福模型(1.4) Rutherford's model
薛定谔方程(1.6) Schrödinger formula
电子排布规则(1.15) rule of electron configurations
能量最低原理 aufbau principle
泡利不相容原理 Pauli exclusion principle
洪特规则 Hund's rule
杂化轨道理论(1.24) hybrid orbital theory
sp3
四面体 tetrahedron
烷 alkane
sp2
平面三角 trigonometria plana
烯 alkene
sp
直线型 linear
炔 alkyne
分子轨道理论(1.36) molecular orbital theroy
成键轨道 bonding MO
反键轨道 antibonding MO
电负性(2.2) electronegativity
元素的性质:吸引核外电子的能力 the intrinsic ability of an atom to attract the shared electrons in a covalent bond
偶极矩(2.7) dipole moment
正、负电荷中心间的距离和电荷中心所带电量的乘积,矢量,单位德拜 DM is defined as the magnitude of the charge Q at either end of the molecular dipole times the distance between the charges
共振结构(2.14) resonancement
若干经典结构式的共振来表达其结构 Resonances of several classical structural formulas to express its structure
互变异构(22.3)
酸碱理论(2.28) acid-base theory
酸碱质子理论(勃朗斯泰德酸碱理论) Brønsted–Lowry acid–base theory
酸碱电子理论(路易斯酸碱理论) Lewis acid-base theory
非共价键相互作用(2.45) non covalent bonding
偶极偶极相互作用 dipole-dipole interactions
色散力 dispersion force
氢键 hydrogen bonding
DNA碱基对之间 DNA base pairs