导图社区 Cambridge IGCS Chemistry Coursebook 2015 Chapter 3 知识点整理
- 20
- 0
- 0
- 举报
Cambridge IGCS Chemistry Coursebook 2015 Chapter 3 知识点整理
这是一篇关于Elements and compounds的思维导图,主要内容包括:Metals, alloys and crystals,The chemical formulae of elements and compounds,Chemical bonding in elements and compounds,The Periodic Table。
编辑于2024-07-24 21:43:59- IGCSE化学
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
Cambridge IGCS Chemistry Coursebook 2015 Chapter 3 知识点整理
社区模板帮助中心,点此进入>>
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
- 相似推荐
- 大纲
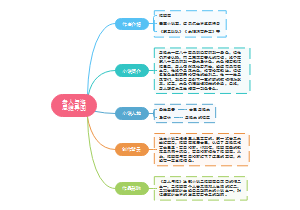
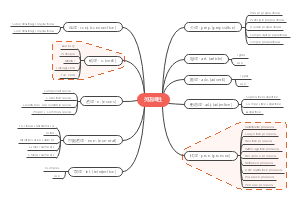
Elements and compounds
Chemical bonding in elements and compounds
Bonding in metals
metallic bonding
Metal atoms more easily lose electrons than gain them. So, they become positive ions. In doing so, they achieve a more stable electron arrangement, usually that of the nearest noble gas.
Bonding in non-metals
covalent bonding
◆ The bond is formed by the sharing of a pair of electrons between two atoms. ◆ Each atom contributes one electron to each bond. ◆ Molecules are formed from atoms linked together by covalent bonds.
Covalent compounds
Chemical bonding in compounds
ionic bounding
Metal plus non-metal compounds are held together by ionic bonding, which results in giant ionic lattices.
Ionic compounds
◆ The electrons involved in the formation of ions are those in the outer shell of the atoms.
◆ Metal atoms lose their outer electrons to become positive ions. In doing so they achieve the more stable electron arrangement of the nearest noble gas.
◆ Generally, atoms of non-metals gain electrons to become negative ions. Again, in doing so, they achieve the stable electron arrangement of the nearest noble gas to them in the Periodic Table.
Features common to ionic bonding
◆ Metal atoms always lose their outer electrons to form positive ions.
◆ The number of positive charges on a metal ion is equal to the number of electrons lost.
◆ Non-metal atoms, with the exception of hydrogen, always gain electrons to become negative ions.
◆ The number of negative charges on a non-metal ion is equal to the number of electrons gained.
◆ In both cases, the ions formed have a more stable electron arrangement, usually that of the noble gas nearest to the element concerned.
◆ Ionic (electrovalent) bonds result from the attraction between oppositely charged ions.
Polytomic (compound) ions
The chemical formulae of elements and compounds
The formulae of ionic compounds
The formulae of covalent compounds
Working out valency
For elements in Groups I–IV, valency = group number
For elements in Groups V–VII, valency = 8 − the group number
Elements in Group VIII / 0 have a valency of 0
This trend in valency with the group number can be seen by looking at typical compounds of the elements of Period 3. You can see that the valency rises to a value of 4 and then decreases to zero as we cross the period.
Naming chemical compounds
◆ If there is a metal in the compound, it is named first.
◆ Where the metal can form more than one ion, then the name indicates which ion is present.
◆ Compounds containing only two elements have names ending in -ide.
◆ Compounds containing a polyatomic ion (usually containing oxygen) have names that end with -ate.
◆ The names of some compounds use prefixes to tell you the number of that particular atom in the molecule. This is useful if two elements form more than one compound;.
◆ The names for the important mineral acids are systematic but are best simply learnt at this stage.
Metals, alloys and crystals
Metal crystals
malleable ductile
grains grain boundaries
Alloys
Alloys are formed by mixing the molten metals together thoroughly and then allowing them to cool and form a solid. Alloying often results in a metal that is stronger than the original individual metals.
the presence of the 'impurity' atoms makes it more difficult for the metal ions to slip over each other. This makes the alloy stronger but more brittle than the metals it is made from.
Ionic crystals
In a metallic crystal, the ions are identical and held together by the mobile electrons. This remains true if one layer is slid against the next. However, pushing one layer against another in an ionic crystal brings ions of the same charge next to each other. The repulsions force the layers apart.
Disruption of an ionic lattice is also brought about by water. Many ionic compounds dissolve in water. Water molecules are able to interact with both positive and negative ions. When an ionic crystal dissolves, each ion becomes surrounded by water molecules. This breaks up the lattice and keeps the ions apart. For those ionic compounds that do not dissolve in water, the forces between the ions must be very strong.
Ions in solution are able to move, so the solution can carry an electric current.
Giant molecular crystals (macromolecules)
Giant molecular crystals are held together by strong covalent bonds. This type of structure is shown by some elements, and also by some compounds.
Moleculart crystals
held together by weak intermolecular forces to form a crystal that is easily broken down by heat. The molecules are then free to move but, unlike the particles in an ionic crystal, they have no charge.
The Periodic Table
Metals and non metals
Metals
1.They are usually solids (except for mercury, which is a liquid) at room temperature. Their melting and boiling points are usually high.
2.They are usually hard and dense.
3.All metals are good conductors of electricity.
4.They are good conductors of heat.
5.Their shape can be changed by hammering (they are malleable). They can also be pulled out into wires (they are ductile).
6.They are grey in colour (except gold and copper). They can be polished.
7.They usually make a ringing sound when struck (they are sonorous).
Non-metals
1.They are solids or gases (except for bromine, which is a liquid) at room temperature. Their melting and boiling points are often low.
2.Most non-metals are softer than metals (but diamond is very hard). Their densities are often low.
3.They are poor conductors of electricity (except graphite, a form of carbon). They tend to be insulators.
4.They are generally poor thermal conductors.
5.Most non-metals are brittle when solid.
6.They vary in colour. They often have a dull surface when solid.
7.They are not sonorous.
Groups and periods in the Periodic Table
main-group elements
transition elements
Electron arrangement and the Periodic Table
◆ Elements in the same group have the same number of electrons in their outer shell.
◆ For the main-group elements, the number of the group is the number of electrons in the outer shell.
◆ The periods also have numbers. This number shows us how many shells of electrons the atom has.
noble gases
unreactive
Trends in groups
GroupⅠ- the alkali metals
Group Ⅶ - the halogens
◆ They are all poisonous and have a similar strong smell. ◆ They are all non-metals. ◆ They all form diatomic molecules (for example Cl2, Br2, I2). ◆ They all have a valency (combining power) of 1 and form compounds with similar formulae, for example hydrogen chloride (HCl), hydrogen bromide (HBr), hydrogen iodide (HI). ◆ Their compounds with hydrogen are usually strong acids when dissolved in water, for example hydrochloric acid (HCl), hydrobromic acid (HBr), hydriodic acid (HI). ◆ They each produce a series of compounds with other elements: chlorides, bromides and iodides. Together these are known as halides. ◆ The halogens themselves can react directly with metals to form metal halides (or salts). ◆ They all form negative ions carrying a single charge, for example chloride ions (Cl−), bromide ions (Br−), iodide ions (I−).
The chemical reactivity of the halogens
oxidising agent
displacement reactions
Group Ⅷ / 0 - the noble gases
uses
Helium ballons Argon light bulbs 'neon' lights
◆ The electron arrangements of the atoms of the noble gases are very stable. ◆ This means that they do not react readily with other atoms. ◆ In many situations where atoms of other elements bond or react chemically, they are trying to achieve that stable arrangement of electrons found in the noble gases.
Trends across a period
The transition elements
◆ They are hard and strong. ◆ They have high density. ◆ They have high melting and boiling points. Two of their distinctive properties: ◆ Many of their compounds are coloured. ◆ They often show more than one valency (variable oxidation state)– they form more than one type of ion. For example, iron can form compounds containing iron(ii) ions (Fe2+) or iron(iii) ions (Fe3+).
The position of hydrogen in the Periodic Table
Glossary
malleable 可展的 Ductile 可延的 Alkali metals 碱金属 Halogens 卤素 Oxidizing agent 氧化剂 Universal Indicator 通用指示剂 Displacement reactions 置换反应 Noble gases 惰性气体 Valency 价 Diatomic molecules 双原子分子 Covalent bonding 共价键 Inert 惰性的 Electrostatic forces 静电力 Grains 晶粒 Grain boundaries 晶界