导图社区 Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
- 14
- 0
- 0
- 举报
Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
编辑于2025-03-08 17:54:46- IGCSE化学
- 化学教材
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
社区模板帮助中心,点此进入>>
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
- 相似推荐
- 大纲
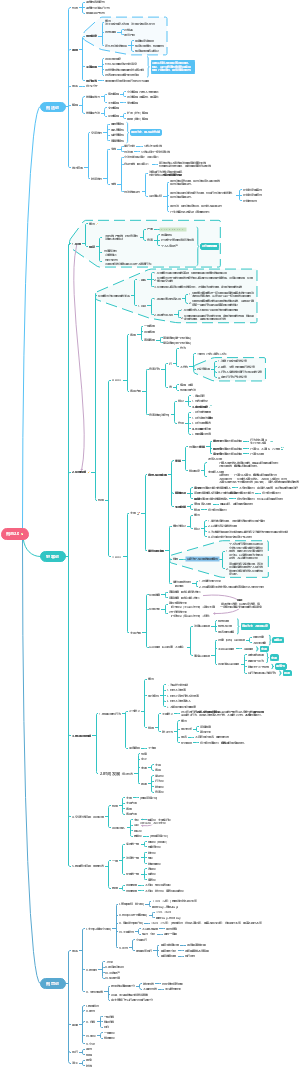
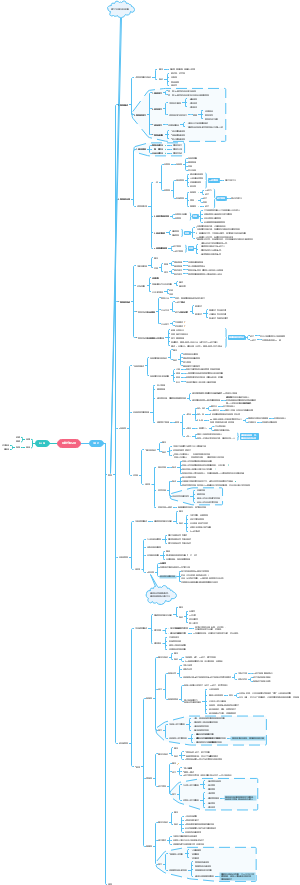
Petrochemicals and polymers
Petroleum and its products
Fossil tuels
•coal •natural gas •petroleum
Formation of petroleum
•high pressure •high temperature •bacteria •over millions of years
Fractional distillation of petroleum
fractions (from distillation): the different mixtures that distil over at different temperatures during fractional distillation
fractionating column: the vertical column that is used to bring about the separation of liquids in fractional distillation
Catalytic cracking
• an alkane with a shorter chain than the original, and a short-chainalkene • or two or more alkenes and hydrogen.
fossil fuels: fuels, such as coal, oil and natural gas, formed underground over geological periods of time from the remains of plants and animals
coal: a black, solid fossil fuel formed underground over geological periods of time by conditions of high pressure and temperature acting on decayed vegetation
natural gas: a fossil fuel formed underground over geological periods of time by conditions of high pressure and temperature acting on the remains of dead sea creatures; natural gas is more than 90% methane
petroleum (or crude oil): a fossil fuel formed underground over many millions of years by conditions of high pressure and temperature acting on the remains of dead sea creatures
non-renewable (finite) resources: sources of energy, such as fossil fuels, and other resources formed in the Earth over many millions of years, which we are now using up at a rapid rate and cannot replace
chemical feedstock: a chemical element or compound which can be used as a raw material for an industrial process making useful chemical products
fractional distillation: a method of distillation using a fractionating column, used to separate liquids with different boiling points
Plastics
plastics: polymers that can be moulded or shaped by the action of heat and pressure
The reuse, recycling and disposal of plastic waste
hydrolysis: a chemical reaction between a covalent compound and water; covalent bonds are broken during the reaction and the elements of water are added to the fragments; can be carried out with acids or alkalis, or by using enzymes
Environmental challenges
ways:
• to reduce the level of plastic packaging wherever possible
• to avoid the use of "single-use plastic' (and the subsequent littering with it)
• to reuse and recycle wherever possible.
alternatives
Incineration can be used to burn plastic waste, although care must be taken not to release toxic fumes into the air. Incineration of PVC, for instance, can release acidic fumes of hydrogen chloride. Open-air burning of plastic occurs at lower temperatures, and normally releases toxic fumes containing dioxins and furans. Controlled high- temperature incineration, above 850°C, must be carried out to break down these toxins. Municipal solid waste incinerators also normally include flue gas treatments to reduce pollutants further.
Disposal in landfill sites suffers from the problem that most plastics are not biodegradable and therefore the plastic waste increasingly fills the space available. This imposes expansion problems on the site and uses up natural resources. Research is being carried out to produce plastics that are biodegradable or photodegradable (broken down by sunlight). However, in all these cases there is the problem of degradation products leaching into the groundwater of a locality used for a landfill site.
Accumulation in oceans. Plastic pollution of the oceans is a major problem that has been highlighted in recent years (and is discussed in the context of other types of pollution in Chapter 17). Increased awareness of this problem has been part of the background to the drive to reduce the level of "single-use plastics' in our shopping and packaging. Images of ocean wildlife and sea birds harmed by interaction with waste plastic debris in the oceans have highlighted the problem. The importance of the problem means that projects to collect plastic waste from the oceans have been started in various regions.
microplastics: small pieces of plastic less than 5mm in length that enter natural ecosystems from a variety of sources including cosmetics, clothing and industrial processes: nurdles and microbeads are different types of microplastic
Polymers
Addition polymerisation
polymer: a substance consisting of very large molecules made by polymerising a large number of repeating units, or monomers
proteins: polymers of amino acids formed by a condensation reaction; they have a wide variety of biological functions
monomer: a small molecule, such as ethene, which can be polymerised to make a polymer
polymerisation: the chemical reaction in which molecules (monomers) join together to form a long-chain polymer
amino acids: naturally occurring organic compounds that possess both an amino (-NH,) group and an acid (-COOH) group in the molecule; there are 20 naturally occurring amino acids and they are polymerised in cells to make proteins
addition polymer: a polymer formed by an addition reaction - the monomer molecules contain a C=C double bond
main features of addition polymers
• all polymers are long-chain molecules made by joining together a large number of monomer molecules
• addition polymerisation involves monomer molecules that contain a C=C double bond
• addition polymers are homopolymers, made from a single monomer
Condensation polymerisation
condensation reaction: a reaction where two or more substances combine together to make a larger compound, and a small molecule is eliminated (given off)
polyamide: a polymer where the monomer units are joined together by amide (peptide) links, e.g. nylon and proteins
polyester: a polymer where the monomer units are joined together by ester links, e.g. PET
Nylon (a polyamide)
PET (a polyester)
condensation polymer: a polymer formed by a condensation reaction, e.g. nylon is produced by the condensation reaction between 1,6-diaminohexane and hexanedioic acid; this is the type of polymerisation used in biological systems to produce proteins, nucleic acids and polysaccharides
amide link (or peptide link): the link between monomers in a protein or nylon, formed by a condensation reaction between a carboxylic acid group on one monomer and an amine group on the next monomer
ester link: the link produced when an ester is formed from a carboxylic acid and an alcohol; also found in polyesters and in the esters present in fats and vegetable oils
Comparing addition and condensation polymerisation
Natural polymers (proteins)