导图社区 Cambridge IGCS Chemistry Coursebook 2023 Chapter 14 知识点整理
Cambridge IGCS Chemistry Coursebook 2023 Chapter 14 知识点整理
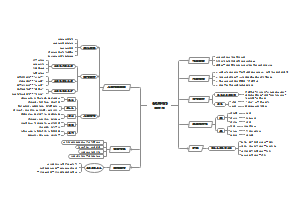
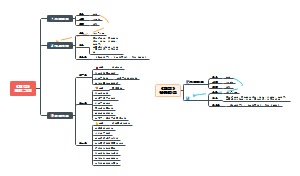
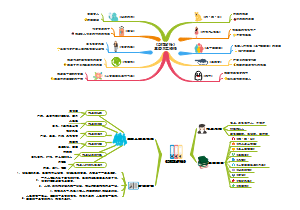
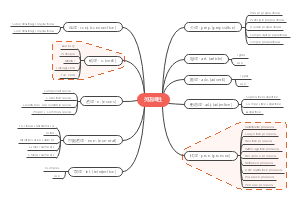
这是一篇关于Metallic elements and alloys的思维导图,主要内容包括:Alloys,Uses of metals,The properties of metals。
编辑于2024-11-23 20:50:28- 金属
- IGCSE化学
- 相似推荐
- 大纲