导图社区 Cambridge IGCS Chemistry Coursebook 2015 Chapter 4 知识点整理
- 16
- 0
- 1
- 举报
Cambridge IGCS Chemistry Coursebook 2015 Chapter 4 知识点整理
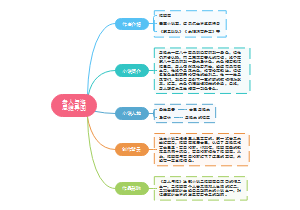
这是一篇关于Chemical reactions的思维导图,主要内容包括:Electrolysis,Redox reactions,Chemical reactions and equations,Types of chemical reaction,Equations for chemical reactions。
编辑于2024-08-01 15:36:31- IGCSE化学
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
Cambridge IGCS Chemistry Coursebook 2015 Chapter 4 知识点整理
社区模板帮助中心,点此进入>>
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
- 相似推荐
- 大纲
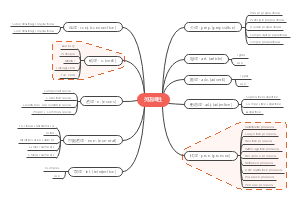
Chemical reactions
Electrolysis
Conductors and insulators
Conductor: a substance that conducts electricity but is not chemically changed in the process.
Supplying electricity
Copper and aluminium with steel core
Electrolytes and non-electrolytes
electrolysis – the breakdown of an ionic compound, molten or in aqueous solution, by the use of electricity.
Metallic conductivity
◆ electrons flow ◆ a property of elements (metals, and carbon as graphite) and alloys ◆ takes place in solids and liquids ◆ no chemical change takes place.
Electrolytic conductivity
◆ ions flow ◆ a property of ionic compounds ◆ takes place in liquids and solutions (not solids) ◆ chemical decomposition takes place.
The movement of ions
electrodes
anode and cathode
◆ positive ions (metal ions or H+ ions) move towards the cathode; they are known as cations
◆ negative ions (non-metal ions) move towards the anode; they are known as anions.
Electrolysis of molten compounds
An electrolytic cell can be used to electrolyse molten compounds.
When a molten ionic compound is electrolysed:
◆ the metal is always formed at the cathode
◆ the non-metal is always formed at the anode.
Industrial electrolysis of molten compounds
Electrolysis of solutions
At the cathode:
◆ The more reactive a metal, the more it tends to stay as ions and not be discharged. The H+ ions will accept electrons instead. Hydrogen molecules will be formed, leaving the ions of the reactive metal in solution.
◆ In contrast, the ions of less reactive metals will accept electrons readily and form metal atoms. In this case, the metal will be discharged, leaving the H+ ions in solution.
At the anode:
◆ If the ions of a halogen (Cl−, Br− or I−) are present in a high enough concentration, they will give up electrons more readily than OH− ions will. Molecules of chlorine, bromine or iodine are formed. The OH− ions remain in solution.
◆ If no halogen ions are present, the OH− ions will give up electrons more easily than any other non-metal anion. Sulfate and nitrate ions are not discharged in preference to OH− ions. When OH− ions are discharged, oxygen is formed.
Electrolysis of concentrated sodium chloride solution
At the cathode, it is the H+ ions that accept electrons, as sodium is more reactive than hydrogen
At the anode, the Cl– ions are discharged more readily than the OH– ions
Electrolysis of acid solutions
Electroplating
Usually the electrodes used in electrolysis are inert (graphite or platinum). However, in electroplating the anode is made of the metal to be plated. It is not inert, and it reacts. The anode decreases in size as it dissolves away.
The basic rules for electroplating an object with a metal M
◆ The object must be made the cathode. ◆ The electrolyte must be a solution of a salt of metal M. ◆ The anode is made of a strip of metal M.
Chemical reactions and equations
Physical change
In a physical change, the substances present remain chemically the same: no new substances are formed.
Physical changes are often easy to reverse. Any mixtures produced are usually easy to separate.
Chemical change
The major feature of a chemical change, or reaction, is that new substance(s) are made during the reaction.
Many reactions, but not all of them, are difficult to reverse.
During a chemical reaction, energy can be given out or taken in
– when energy is given out, the reaction is exothermic
– when energy is taken in, the reaction is endothermic
There are many more exothermic reactions than endothermic reactions.
Equations for chemical reactions
Balanced symbol equations
law of conservation of mass – the total mass of all the products of a chemical reaction is equal to the total mass of all the reactants.
It is important to remember that:
·You cannot change the formulae of the substances themselves when balancing equations. These are fixed by the nature of the atoms and their bonding.
·The only things that you can change when balancing are the numbers in front of the formulae.
Types of chemical reaction
Synthesis and decomposition
Synthesis
reactions occur where two or more substances react together to form just one product.
Phtotsynthesis
Decomposition
reactions have just one reactant, which breaks down to give two or more simpler products.
Neutralisation and precipitation
Neutralisation
When acids react with bases or alkalis, their acidity is destroyed. They are neutralised and a salt is produced.
Precipitation
the sudden formation of a solid, either when two solutions are mixed or when a gas is bubbled into a solution.
Displacement reaction
子主题
a more reactive element will displace a less reactive one from a solution of one of its compounds.
Combustion, oxidation and reduction
Combustion
the reaction of a substance with oxygen causing the release of energy. The reaction is exothermic and often involves a flame.
burning – combustion in which a flame is produced.
Oxidation and reduction
If a substance gains oxygen during a reaction, it is oxidised.
Corrosion and rancidity
Oxidising agent
If a substance loses oxygen during a reaction, it is reduced.
Reducing agent
Redox reactions
Ionic equations
State symbols
s: solid l: liquid g: gass aq: aqueous solution
Redox reactions
Oxidation is the loss of electrons.
Oxidation is the increase in oxidation state of an atom or ion.
Reduction is the gain of electrons.
Reduction is the decrease in oxidation state of an atom or ion.
Tests for oxidising and reducing agents
Glossary
Chemiluminescence 化学发光 Endothermic 吸热的 Exothermic 放热的 Law of conservation of mass 质量守恒定律 Thermal decomposition 热分解 Neutralisation 中和 Precipitation 沉淀 Displacement 置换 Combustion 氧化(燃烧) Oxidation 氧化 Reduction 还原 Reducing agent 还原剂 Redox 氧化还原反应 Rancidity 腐败 Electrolysis 电解 Electrolytes 电解质 Electrodes 电极 Cathode 负极 Anode 正极 Electroplating 电镀