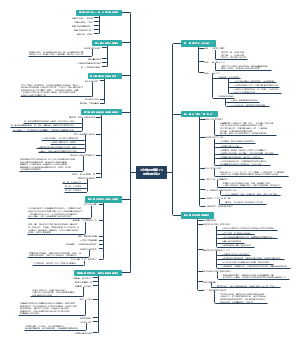
导图社区 ICH 指导原则
- 2
- 0
- 0
- 举报
ICH 指导原则
这是一篇关于ICH 指导原则的思维导图,主要内容包括:质量(Quality Guidelines),安全性(Safety Guidelines),有效性(Efficacy Guidelines),多学科(Multidisciplinary Guidelines)。
编辑于2025-10-11 13:36:13- 指导原则
- 技术文件
- 质量指导原则
- 领导梯队模型
这是一篇关于领导梯队模型的思维导图,主要内容包括:领导力转型“三板斧”,2. 管理他人(一线经理),4. 管理职能部门(事业部副总经理),6. 管理业务群组(集团高管,集团副总裁),7. 管理全集团(首席执行官),5. 管理事业部(事业部总经理),3. 管理经理人员(部门总监),1. 自我管理(个人贡献者)。
- ICH 指导原则
这是一篇关于ICH 指导原则的思维导图,主要内容包括:质量(Quality Guidelines),安全性(Safety Guidelines),有效性(Efficacy Guidelines),多学科(Multidisciplinary Guidelines)。
- 药品注册分类
这是一篇关于药品注册分类的思维导图,汇总了不同类型药品的注册分类标准,主要内容包括:化学药品,中药、天然产物,生物制品,有助于理解药品注册的相关要求和规范。
ICH 指导原则
社区模板帮助中心,点此进入>>
- 领导梯队模型
这是一篇关于领导梯队模型的思维导图,主要内容包括:领导力转型“三板斧”,2. 管理他人(一线经理),4. 管理职能部门(事业部副总经理),6. 管理业务群组(集团高管,集团副总裁),7. 管理全集团(首席执行官),5. 管理事业部(事业部总经理),3. 管理经理人员(部门总监),1. 自我管理(个人贡献者)。
- ICH 指导原则
这是一篇关于ICH 指导原则的思维导图,主要内容包括:质量(Quality Guidelines),安全性(Safety Guidelines),有效性(Efficacy Guidelines),多学科(Multidisciplinary Guidelines)。
- 药品注册分类
这是一篇关于药品注册分类的思维导图,汇总了不同类型药品的注册分类标准,主要内容包括:化学药品,中药、天然产物,生物制品,有助于理解药品注册的相关要求和规范。
- 相似推荐
- 大纲
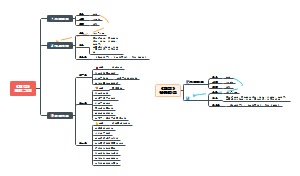
ICH 指导原则
质量(Quality Guidelines)
Q1 Stability/稳定性
Q1A(R2): Stability Testing of New Drug Substances and Products Q1A(R2):新原料药和制剂的稳定性试验
Q1B: Stability Testing: Photostability Testing of New Drug Substances and Products Q1B:稳定性试验:新原料药和制剂的光稳定性试验
Q1C: Stability Testing for New Dosage Forms Q1C:新剂型的稳定性试验
Q1D: Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products Q1D:新原料药和制剂稳定性试验的括号法和矩阵法设计
Q1E: Evaluation for Stability Data Q1E:稳定性数据的评价
Q2 Analytical Validation/分析方法验证
Q2(R2):Validation of Analytical Procedures Q2(R2):分析方法验证
Q3A - Q3D Impurities/杂质
Q3A(R2): Impurities in New Drug Substances Q3A(R2):新原料药中的杂质
Q3B(R2): Impurities in New Drug Products Q3B(R2):新药制剂中的杂质
Q3C(R9):Impurities: Guideline for Residual Solvents Q3C(R9):杂质:残留溶剂的指导原则
Q3D(R2): Guideline for Elemental Impurities Q3D(R2):元素杂质指导原则
Q4 - Q4B Pharmacopoeias/药典
Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions Q4B:ICH区域所用药典文本的评价和建议
Q4B Frequently Asked Questions Q4B:常见问题与解答
Q4B Annex 1 (R1): Residue on Ignition/Sulphated Ash General Chapter Q4B附录1(R1):关于灼烧残渣/灰分 常规篇
Q4B Annex 2 (R1): Test for Extractable Volume of Parenteral Preparations General Chapter Q4B附录2(R1):关于注射剂可提取容量测试 常规篇
Q4B Annex 3 (R1): Test for Particulate Contamination: Sub-Visible Particles General Chapter Q4B附录3(R1):关于颗粒污染物测试:不溶性微粒 常规篇
Q4B Annex 4A (R1): Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests General Chapter Q4B附录4A(R1):非无菌药品的微生物检查:微生物计数试验 常规篇
Q4B Annex 4B (R1): Microbiological Examination of Non-Sterile Products Tests for Specified Micro-Organisms General Chapter Q4B附录4B(R1):非无菌产品的微生物检查—特定微生物 常规篇
Q4B Annex 4C (R1): Microbiological Examination of Non-Sterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use General Chapter Q4B附录4C(R1):非无菌产品的微生物检查:药物制备以及药物使用物质的接受标准 常规篇
Q4B Annex 5 (R1): Disintegration Test General Chapter Q4B附录5(R1):崩解试验 常规篇
Q4B Annex 6 Uniformity of Dosage Units General Chapter Q4B附录6: 统一剂量单位 常规篇
Q4B Annex 7 (R2): Dissolution Test General Chapter Q4B附录7(R2): 溶出试验 常规篇
Q4B Annex 8 (R1): Sterility Test General Chapter Q4B附录8(R1): 无菌试验 常规篇
Q4B Annex 9 (R1): Tablet Friability General Chapter Q4B附录9(R1):片剂易碎性 常规篇
Q4B Annex 10 (R1): Polyacrylamide Gel Electrophoresis General Chapter Q4B附录10(R1):聚丙烯酰胺凝胶电泳 常规篇
Q4B Annex 11: Capillary Electrophoresis General Chapter Q4B附录11:毛细管电泳 常规篇
Q4B Annex 12: Analytical Sieving General Chapter Q4B附录12:分析筛选 常规篇
Q4B Annex 13: Bulk Density and Tapped Density of Powders General Chapter Q4B附录13:粉末的堆密度和振实密度
Q4B Annex 14: Bacterial Endotoxins Test General Chapter Q4B附录14:细菌内毒素试验 常规篇
Q5A - Q5E Quality of Biotechnological Products/生物技术产品质量
Q5A(R2):Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin Q5A(R2):来源于人或动物细胞系生物技术产品的病毒安全性评价
Q5B: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products Q5B:源自重组DNA技术的蛋白质产品的表达载体分析
Q5C: Stability Testing of Biotechnological/Biological Products Q5C:生物技术生物制品质量:生物技术/生物制品稳定性试验
Q5D: Derivation and Characterisation of Cell Substrates Used for Production of BiotechnologicalBiological Products Q5D: 用于生产生物技术/生物产品的细胞底物的起源和特征描述
Q5E: Comparability of BiotechnologicalBiological Products Subject to Changes in their Manufacturing Process Q5E:生物技术产品/生物制品在生产工艺变更前后的可比性
Q6A- Q6B Specifications/规格
Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances Q6A:质量标准:新原料药和新药制剂的检测方法和可接受标准:化学药物
Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products Q6B:质量规格:生物技术/生物产品的检验程序和可接收标准
Q7 Good Manufacturing Practice/GMP
Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7:原料药的药品生产质量管理规范指南
Q7 Questions and Answers Q7问答:原料药的药品生产质量管理规范指南问答
Q8 Pharmaceutical Development/药物研发
Q8(R2): Pharmaceutical Development Q8(R2):药品研发
Q8, Q9 and Q10 Questions & Answers (R4) 关于Q8、Q9和Q10的问与答(R4)
Q9 Quality Risk Management/质量风险管理
Q9(R1):Quality Risk Management Q9(R1):质量风险管理
Q10 Pharmaceutical Quality System/药物质量体系
Q10: Pharmaceutical Quality System Q10:药品质量体系
Q11 Development and Manufacture of Drug Substances/化学药品的研发与生产
Q11: Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11:原料药开发和生产(化学实体和生物技术/生物实体药物)
Q11:Questions and Answers Q11问答:原料药开发和生产(化学实体和生物技术/生物实体药物)问答
Q12 Techinical And Regulatory Considerations For Pharmaceutical Product Lifecycle Management药品生命周期管理的技术和监管考虑
Q12:Techinical And Regulatory Considerations For Pharmaceutical Product Lifecycle Management Q12:药品生命周期管理的技术和监管考虑
Q12 Annexes Q12附件
Q13:Continuous Manufacturing of Drug Substances and Drug Products原料药和制剂的连续制造
Q13:Continuous Manufacturing of Drug Substances and Drug Products Q13:原料药和制剂的连续制造
Q14:Analytical Procedure Development分析方法开发
Q14:Analytical Procedure Development Q14:分析方法开发
安全性(Safety Guidelines)
S1A - S1C Carcinogenicity Studies/致癌性研究
S1A: Need for Carcinogenicity Studies of Pharmaceuticals S1A:药物致癌性试验必要性指导原则
S1B: Testing for Carcinogenicity of Pharmaceuticals S1B:药物致癌性试验
S1B(R1):TESTING FOR CARCINOGENICITY OF PHARMACEUTICALS S1B(R1):药物致癌性试验
S1C(R2): Dose Selection for Carcinogenicity Studies of Pharmaceuticals S1C(R2):药物致癌性试验的剂量选择
S2 Genotoxicity Studies/基因毒性研究
S2(R1): Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2(R1):人用药物遗传毒性试验和结果分析指导原则
S3A - S3B Toxicokinetics and Pharmacokinetics/毒代动力学和药代动力学
S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies S3A:毒代动力学指导原则说明:毒性研究中的全身暴露量评价
S3A Implementation Working Group Questions and Answers S3A 问答 毒代毒代动力学指导原则说明:毒性研究中的全身暴露量评价-聚焦于微量采样
S3B: Pharmacokinetics Guidance for Repeated Dose Tissue Distribution Studies S3B:药代动力学:重复给药的组织分布研究指导原则
S4 Toxicity Testing/毒性试验
S4: Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing) S4:动物慢性毒性试验的期限(啮齿类和非啮齿类)
S5 Reproductive Toxicology/生殖毒性
S5(R2):Detection of Toxicity to Reproduction for Medicinal Products & Toxicity to Male Fertility S5(R2): 检测药品的生殖毒性以及对雄性生殖能力的毒性
S5(R3): Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals S5(R3):人用药物生殖与发育毒性检测
S6 Biotechnological Products/生物技术产品
S6(R1): Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals S6(R1):生物制品的临床前安全性评价
S7A - S7B Pharmacology Studies/药理学研究
S7A: SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS S7A:人用药品安全药理学试验指导原则
S7B: The Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals S7B:人用药品延迟心室复极化(QT间期延长)潜在作用的非临床评价指导原则
S8 Immunotoxicology Studies 免疫毒理学研究
S8: Immunotoxicity Studies for Human Pharmaceuticals S8:人用药物免疫毒性研究
S9 Nonclinical Evaluation for Anticancer Pharmaceuticals/抗癌药物的非临床评价
S9: Nonclinical Evaluation for Anticancer Pharmaceuticals S9:抗肿瘤药物非临床评价指导原则
S9 Implementation Working Group Questions and Answers S9:抗肿瘤药物非临床评价指导原则问答
S10 Photosafety Evaluation/光安全性评价
S10: Photosafety Evaluation of Pharmaceuticals S10:药物光安全评价
S11 Nonclinical Safety Testing In Support of Development of Paediatric Pharmaceuticals/儿科用药
S11:Nonclinical Safety Testing In Support of Development of Paediatric Pharmaceuticals S11:支持儿科用药开发的非临床安全性评价
S12:Nonclinical Biodistribution Considerations For Gene Therapy Products/基因治疗产品非临床生物分布的考虑
S12:Nonclinical Biodistribution Considerations For Gene Therapy Products S12:基因治疗产品非临床生物分布的考虑
有效性(Efficacy Guidelines)
E1 Clinical Safety for Drugs used in Long-Term Treatment/长期使用的药物的临床安全性
E1: The extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-term Treatment of Non-life-threatening Conditions E1:人群暴露程度:评估非危及生命性疾病长期治疗药物的临床安全性
E2A - E2F Pharmacovigilance/药物警戒性
E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A: 临床安全性数据管理:快速报告的定义和标准
E2B(R3):Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) E2B(R3) Data Elements and Message Specification E2B(R3):个例安全报告(ICSR)电子传输执行指导原则 E2B(R3)数据元素和信息规范元素 (中文版:征求意见稿)
E2B(R3) QA document_v2.4 E2B(R3) 问答文件(版本2.4)
E2C(R2): Periodic Benefit-Risk Evaluation Report E2C(R2): 定期获益—风险评估报告
E2C(R2) Implementation Working Group Questions & Answers E2C(R2)实施工作组 问答部分
E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D: 上市后安全性数据的管理:快速报告的定义和标准(中文版:征求意见稿)
E2E: Pharmacovigilance Planning E2E:药物警戒计划
E2F:Example DSUR – Phase III Investigational Drug E2F:研发期间安全性更新报告示例
E2F: Development Safety Update Report E2F:研发期间安全性更新报告
E3 Clinical Study Reports/临床研究报告
E3: Structure and Content of Clinical Study Reports E3:临床研究报告的结构与内容
E3 Questions & Answers (R1) : Structure and Content of Clinical Study Reports E3:临床研究报告的结构和内容问与答(R1)
E4 Dose-Response Studies/剂量反应研究
E4: Dose-Response Information to Support Drug Registration E4:药品注册所需的量效关系信息
E5 Ethnic Factors/种族因素
E5(R1): Ethnic Factors in the Acceptability of Foreign Clinical Data E5(R1):接受国外临床试验数据的种族因素
E5 Implementation Working Group Questions & Answers (R1) E5:接受国外临床试验数据的种族因素问答(R1)
E6 GCP/药物临床试验管理规范
E6(R1): Guideline for Good Clinical Practice E6(R1):药物临床试验管理规范指导原则
E6(R2):Integrated Addendum to Good Clinical Practice (GCP) E6(R2):药物临床试验管理规范综合附录
E7 Clinical Trials in Geriatric Population/老人中开展的临床试验
E7: Studies in Support of Special Populations: Geriatrics E7:特殊人群的研究:老年医学
E7 Questions & Answers E7:特殊人群的研究:老年医学问答
E8 General Considerations for Clinical Trials/临床试验的一般性考虑
E8(R1): General Considerations for Clinical Trials E8(R1):临床试验的一般考虑
E9 Statistical Principles for Clinical Trials/临床试验的统计原则
E9: Statistical Principles for Clinical Trials E9:临床试验的统计学原则
E9(R1): Addendum on E stimands and Sensitivity Analysis in Clinical Trials E9(R1):临床试验中的估计目标与敏感性分析(E9指导原则增补文件)
E10 Choice of Control Group in Clinical Trials/试验中对照组的选择
E10: Choice of Control Group and Related Issues in Clinical Trials E10:临床试验中对照组的选择和相关问题
E11 Clinical Trials in Pediatric Population/儿童人群临床研究
E11(R1): Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population E11(R1):用于儿科人群的医学产品的药物临床研究
E11A:Pediatric Extrapolation E11A:儿科外推
E12 Clinical Evaluation by Therapeutic Category/根据治疗类别进行临床评价
E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs E12A:抗高血压新药临床评价原则
E14 Clinical Evaluation of QT/QT临床评价
E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs E14:非抗心律失常药物QT/QTc间期延长及致心律失常潜力的临床评价
E14 Implementation Working Group Questions & Answers (R3) E14 实施工作组 问答部分(R3)
E14/S7B: Clinical and Nonclinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential Questions and Answers E14/S7B: QT/QTc 间期延长及潜在致心律失常作用的临床和非临床评价问答
E15 Definitions in Pharmacogenetics/Pharmacogenomics/药物基因组学以及遗传药理学相关定义
E15: Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories E15:基因组生物标志物、药物基因组学、遗传药理学、基因组数据和样本编码分类的定义
E16 Qualification of Genomic Biomarkers/基因组生物标志物的合格条件
E16: Biomarkers Related to Drug or Biotechnology Product Development: Context, Structure and Format of Qualification Submissions E16:药物或生物技术产品开发相关的生物标志物:资格认定申请的背景资料、结构和格式
E17 Multi-Regional Clinical Trials/多地区临床试验
E17: General principle on planning and Designing Multi-Regional Clinical Trials E17:多区域临床试验计划与设计的一般原则
E18 Genomic Sampling/基因组取样
E18: Genomic Sampling and Management of Genomic Data E18:基因组采样和基因组数据管理指导原则(中文翻译公开征求意见稿)
E19:A Selective Approach To Safety Data Collection In Specific Late-Stage Pre-approval Or Post-Approval Clinical Trials/在特定的上市前后期或上市后临床试验中选择性收集安全性数据
E19:A Selective Approach To Safety Data Collection In Specific Late-Stage Pre-approval Or Post-Approval Clinical Trials E19:在特定的上市前后期或上市后临床试验中选择性收集安全性数据
多学科(Multidisciplinary Guidelines)
M1 MedDRA Terminology 监管活动医学词典
MedDRA Points to Consider Companion Document MedDRA ® 数据检索和展示: 考虑要点
MedDRA Term Selection: Points to Consider MedDRA ® 术语选择: 考虑要点
MedDRA Best Practices MedDRA ® 最佳规范
M2 Electronic Standards 电子标准
M2:Electronic Standards for the Transfer of Regulatory Information Final Concept Paper M2:监管信息电子传输标准 最终概念文件
Electronic Standards for the Transfer of Regulatory Information (ESTRI) General Recommendation - Procedure 监管信息电子传输标准 一般性建议-程序
Electronic Standards for the Transfer of Regulatory Information (ESTRI)-Gateway 监管信息电子传输标准 一般性建议-ESTRI网关
Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – PDF 监管信息电子传输标准 文件格式建议-PDF
Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – XML 监管信息电子传输标准 文件格式建议-XML
Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – PDF/A 监管信息电子传输标准 文件格式建议-PDF/A
Electronic Standards for the Transfer of Regulatory Information (ESTRI)File Format Recommendation – DOCX 监管信息电子传输标准 文件格式建议-DOCX
Electronic Standards for the Transfer of Regulatory Information Controlled Vocabularies Recommendation - Genericode 监管信息电子传输标准 受控词汇建议-通用编码
Electronic Standards for the Transfer of Regulatory Information Information Transfer Recommendation – EDIINT V3.0 监管信息电子传输标准 信息传输建议-EDIINT V3.0
Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Integrity – MD5 监管信息电子传输标准 文件完整性-MD5
Electronic Standards for the Transfer of Regulatory Informaation (ESTRI) File Integrity Recommendation - SHA-256 监管信息电子传输标准 文件完整性建议-SHA-256
M2:Glossary of Terms and Abbreviations M2:术语和缩略语词汇表
M2:File Format Criteria M2:文件格式标准
Use of OIDs & UUIDs in ICH Messages OID和UUID在ICH消息中的应用
M3 Nonclinical Safety Studies 非临床研究
M3(R2) Questions and Answers (R2) M3(R2)问答 (R2)
M3(R2): Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals M3(R2):支持药物进行临床试验和上市的非临床安全性研究指导原则
M4 : The Common Technical Document 通用技术文件
M4 (R4): Organization of the Common Technical Document for the Registration of Pharmaceuticals for Human Use M4(R4):人用药物注册通用技术文档的组织(中文版:征求意见稿)
M4 Implementation Working Group Questions & Answers (R3) M4执行工作组问答(R3)(中文版:征求意见稿)
The Common Technical Document for the Registration of Pharmaceuticals for Human Use: Quality – M4Q(R1) M4Q(R1):人用药物注册通用技术文档:药学部分(中文版:征求意见稿)
M4Q Implementation Working Group Questions & Answers (R1) M4Q执行工作组问答(R1)(中文版:征求意见稿)
The Common Technical Document for the Registration of Pharmaceuticals for Human Use: Safety – M4S(R2) M4S(R2):人用药物注册通用技术文档:安全性部分(中文版:征求意见稿)
M4S Implementation Working Group Questions & Answers (R4) M4S执行工作组问答 (R4)(中文版:征求意见稿)
Efficacy- M4E(R2) M4E(R2):人用药物注册通用技术文档:有效性部分(中文版:征求意见稿)
M4E Implementation Working Group Questions & Answers (R4) M4E执行工作组问答(R4)(中文版:征求意见稿)
M5 Data Elements and Standards for Drug Dictionaries 药物词典的数据要素和标准
The Re-development of the Standard for E2B(R3) and the Development of Standards for the Identification of Medicinal Products (IDMP)(ICH M5) ICH M5: E2B(R3)标准的再制定及医药产品鉴定标准的制定
ICH E2B(R3) Implementation Working Group ICH E2B(R3) Guideline: Electronic Transmission of Individual Case Safety Reports (ICSRs) E2B(R3)实施工作组 个例病例安全报告的电子传输 问答部分
Appendix I (B) to the Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) 个例病例安全报告的电子传输实施指南附录 I (B)
Appendix I (G) to the Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) 个例病例安全报告的电子传输实施指南附录 I (G)
Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) 个例病例安全报告的电子传输实施指南
M6 Gene Therapy 基因治疗
Final Concept Paper M6: Guideline on Virus and Gene Therapy Vector Shedding and Transmission M6: 病毒和基因治疗载体的脱落和传播 终版概念文件
General Principles to Address Virus and Vector Shedding 解决病毒和基因治疗载体脱落的基本原则
An inventory of shedding data from clinical gene therapy trials 临床基因疗法试验脱落数据目录
Final Business Plan M6: Guideline on Virus and Gene Therapy Vector Shedding and Transmission M6: 病毒和基因治疗载体的脱落和传播 终版业务计划
M7 Genotoxic Impurities 遗传毒性杂质
M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7:评估和控制药物中的DNA活性(致突变)杂质以限制潜在的致癌风险
M7(R1): Addendum to M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R1):评估和控制药物中DNA反应性(致突变)杂质以限制潜在致癌风险
M7(R2):Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R2):评估和控制药物中DNA反应性(致突变)杂质以限制潜在致癌风险
M7(R2):Questions and Answers M7(R2):问答文件
Addendum to M7(R2) :Application of The Principles of the ICH M7 Guideline to Calculation of Compound-Specific Acceptable Intakes M7(R2)附录:ICH M7原则在化合物可接受摄入量计算中的应用
M8 Electronic Common Technical Document (eCTD) 电子通用技术文件
Electronic Common Technical Document Specification V3.2.2 电子通用技术文件规范 V3.2.2
M8 : Electronic Common Technical Document Concept Paper M8: 电子通用技术文件 概念文件
ICH M8 EWG/IWG Work Plan M8: 电子通用技术文件 工作计划
Support Documentation for M8: eCTD EWG eCTD v4.0 Implementation Package v1.2 M8:eCTD专家工作组eCTD v4.0实施包 v1.2 支持性证明文件
Orientation Material forM8: eCTD EWG eCTD v4.0 Implementation Package v1.2 M8:eCTD专家工作组eCTD v4.0实施包 v1.2 培训材料
ICH Electronic Common Technical Document (eCTD) v4.0 Implementation Guide v1.2 ICH eCTD v4.0 实施指南 v1.2
eCTD v4.0 Implementation Package v1.2 eCTD v4.0 实施包 v1.2
USFDA eCTD v4.0 Implementation Package History v1.1 美国FDA eCTD v4.0 实施包历史 v1.1
USFDA Module 1 Electronic Common Technical Document (eCTD) v4.0 Implementation Guide v1.1 美国FDA 模块1 eCTD v4.0 实施指南 v1.1
ICH eCTD v4.0 Requirements ICH eCTD v4.0 要求
ICH M8 Expert Working Group Specification for Submission Formats for eCTD eCTD提交格式规范
Change Control Process for the eCTD eCTD变更控制过程
Request for change 请求变更表
M9 Biopharmaceutics Classification System-based Biowaivers 基于生物药剂学分类系统的生物豁免
M9: Biopharmaceutics Classification System-based Biowaivers M9:基于生物药剂学分类系统的生物等效性豁免
M9 Questions and Answers M9问答文件
M10 Bioanalytical Method Validation and Study Sample Analysis 生物分析方法验证及样品分析
M10 Bioanalytical Method Validation and Study Sample Analysis M10:生物分析方法验证及样品分析
M10 Questions and Answers(Q&As) M10问答文件
M10 Frequently Asked Questions (FAQs) M10常见问题解答文件
M12 Drug Interaction Studies
M12:Drug Interaction Studies M12:药物相互作用研究
M12:Drug Interaction Studies Q&A M12:药物相互作用研究问答文件