导图社区 The Cell killer Cells
- 19
- 0
- 0
- 举报
The Cell killer Cells
这是一篇关于The Cell killer Cells的思维导图,主要内容有Symdrom、severity、Development、epidemiology terms。
编辑于2022-06-11 15:42:11- NK cells
- CTL
- eachother
- Cell killer …
- 相似推荐
- 大纲
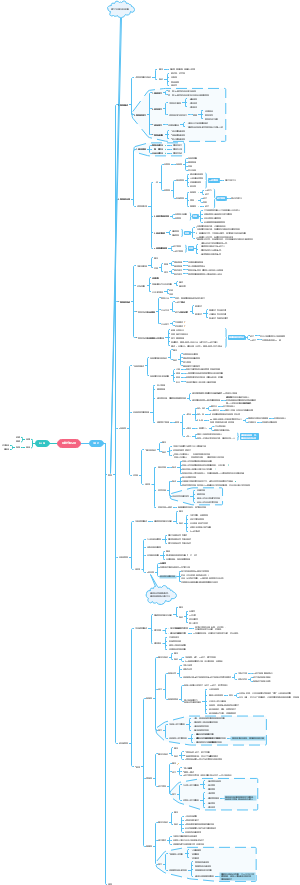
The Cell killer Cells
NK(Natural killer) cells
precoursor
Common lymphoid progenitor cells
which can differentiate into
T
B
NK
10% of lymphocytes in blood
differentiation & mature locations
bone marrow
lymph node
spleen
tonsils
thymus
tragets
MHC/antibody dependent
criteria
Missing MHCI
as self marker
The dangerous cells missing MHCI can only be eliminated by NK cells.
NK cells preferentially kill cells with low level of MHCI
evolutionary explanation
the chronic down-regulation of MHC I molecules, which makes affected cells invisible to T cells, allowing them to evade T cell-mediated immunity. NK cells apparently evolved as an evolutionary response to this adaptation (the loss of the MHC eliminates CD4/CD8 action, so another immune cell evolved to fulfill the function).
viral infected cells
bacteria infected cells
malignent cells
MHC/antibody independent
any stressed cells
time
3 days after infection
surface
human
Markers
CD16
CD58
CD8(80%)
Receptors
activating receptors
the final effect to the NK is the sum of all
most of those receptors are absence in CTL
NCR(Natural cytotoxicity receptors)
ligands
things that indicate the infection of the target
effect
directly induce target apoptosis
release of interferon γ
organization
monomer
family
revolutionary distance
CD94 : NKG2
organization
heterodimer
family
C-type lectin family receptor
revolutionary distance
ligands
non-classical (also non-polymorphic) MHC I molecules
effect
presence in both human and mouse
CD16(Fc Y RIII)
organization
monomer
family
revolutionary distance
ligands
Fc region of IgG
effect
play a role in antibody-dependent cell-mediated cytotoxicity (ADCC)
The power of ADCC depends on the affinity of the Fc receptor expressed on NK cells
This affinity is determined by the nucleotide status in position 158 of the gene
Receptors involved in the lysis of tumor cells
NKG2D
disulfide-linked homodimer
ligands recognized
proteins specifically expressed on tumor cells
ULBP
MICA
some tumor cells(prostate cancer) can produce NKG2D soluble ligands to evade NK
false NK response
compete the NKG2D
NKp44, NKp46, NKp30, and DNAM.
inhibitory receptors
recognize MHC class I alleles
Many are MHC dependent receptors
ligands
MHCI
effect
induce target apoptosis with an alternative pathway
revolutionary distance
ligands
effect
KIR (Killer-cell immunoglobulin-like receptors)
organization
family
Ig-like extracellular domain receptors
revolutionary distance
more recently evolved
present in non-human primates
ligands
are the main receptors for both classical MHC I (HLA-A, HLA-B, HLA-C) and also non-classical HLA-G in primates.
effect
so the normal cell expressing MHC will binds to KIR and inhibits NK killing
ILT or LIR (leukocyte inhibitory receptors)
organization
family
Ig receptor family
revolutionary distance
ligands
effect
Ly49
organization
homodimer
family
C-type lectin family receptor
revolutionary distance
ancient
ligands
Classicle(polymorphic)MHCI
effect
psuedogenic in human?
multigenic in mouse
absence
mostly
lack antigen-specific cell surface receptors and therefore are part of innate immunity
TCR
BCR
CD3
apoptosis mechanism
NK first close approximate the target
Then it can initiates
Cytolytic granule mediated cell apoptosis
The proteins below are stored within the granules of NKs
perforins
perforins first insert into membrane of target cell, and polymerize into a aquouse channel, which can allows granzymes and other proteins to get in
granzymes
protease
granzymes enter the pore and activate caspases in target cells
activation of caspases induce apoptosis
completely distroy anything inside
can eliminate virions
or osmotic cell lysis
cannot distroy everything
can release virions
Antibody-dependent cell-mediated cytotoxicity (ADCC)
anitobies oposonize the infected cells.
FcϒRIII (CD16) receptors on NK recognize antigen recognized antibody
this activates NK cell, release cytolytic granules
cytokines released
IFN-γ
activates macrophages
TNFα
promote direct NK tumor cell killing
interleukin (IL-10)
immuno-suppressors
α-defensin
can disrupt bacteria cell wall
only release when activated
the aim is to control viral infection
they can activates macrophages, dendritic cells, neutrophils
subsequently activates adaptive immunity
cytokines sensed
Cytokine-induced NK and CTL activation
cytokines are stress molecules released by cells upon viral infection
Cytokines can be interferons or macrophage-derived cytokines
cytokines that can activates NK
IL-12, IL-15, IL-18, IL-2, and CCL5
IFN-Y
secreted by NKT
role
innate immunity
mostly
adaptive immuhave
essential to form immunological memory
NK cells have been found to undergo expansion, contraction, memory maintenance and recallnity
CTL (Cytotoxic T lymphocytes)
precoursor
hematopoitic stem cells
migrate from the bone marrow to the thymus
CD8+ cells
requires stimulation of antigen presentation to mature
Two signals modle for CTL activation
TCR
most cases
APC
peptide-bound MHC class I molecule
other coreceptors
CD8
There is a second interaction between the CD8 coreceptor and the class I MHC molecule to stabilize this signal.
CD28
proteins on T cell surface
APC
either CD80 or CD86 (also called B7-1 and B7-2)
CD80 and CD86 are known as costimulators for T cell activation. This second signal can be assisted (or replaced) by stimulating the TC cell with cytokines released from helper T cells.
not all the CD8+ cells are CTL, but some of imature CD8+ can release cytokines.
onces a CTL is activated, it undergoes colonal expansion.
with the help of cytokines IL2
growth and differentiation factor for all the T cells
the expansion of the specific T cell will find and target the antigen presented somatic cells in the body
naive form activated by MHC I bonded antigen
by both professional/non professional APCs
The detailed differentiation and activation process of T cells in thymus
The coreceptors double positive TCR have both CD8 and CD4, and it will undergoes 2 selection stages
positive selection
double-positive T cells that bind too weakly to MHC-presented self antigens undergo apoptosis because of their inability to recognize MHC-protein complexes.
negative selection
double-positive T cells that bind too strongly to MHC-presented self antigens undergo apoptosis because they could otherwise become autoreactive, leading to autoimmunity.
The cells survived two round of selections become single positive T cells
CD4+
their TCR recognizes an MHC class II-presented antigen
CD8+
their TCR recognizes an MHC class I-presented antigen
mature by the activation with a class I-restricted antigen
becomes cytotoxic T cells
VDJ recombination
the DNA in millions of white blood cells in the bone marrow is shuffled to create cells with unique receptors
The receptors that can bind to the self-antigen will lead to apoptosis.
targets
viral infected cells
bacteria infected cells
malignent cells
physically damaged cells
dysfunctioned somatic cells
all the alter-self cells
surface
T-cell receptors(TCR)
can recognize antigens, in this case mostly are MHCI binded antigens.
structure
beta chain
The beta chain DNA first get VDJ recombination in thymus within the hematopoitic stem cells.
so, the TCR get its developmental form/pre-TCR.
alpha chain
The alpha chain DNA will get recombined if the beta chain recombination is successful.
then the fully functioned alpha-beta TCR will be produced.
This TCR virtually has millions of possible structures and can virtually binds to any proteins
gamma and delta chains(some TCR may have)
expressed on cells in epicilial tissues
against non-protein antigens
CD8
a coreceptor for TCR binding with MHC I, it is essential for the antigen presentation/atigen specific activation
it binds with the constant portion of MHC I
Molecular mechanisms to initiate apoptosis
Perforin-induced apopotosis
they are together called cytotoxin
perforin+A&B Granzymes
Granzymes have serine protease activity
they trigure the caspase cascade in cytoplasm
caspase cascade involves a serious of cysteine protease
this cascade finally leads to apoptosis
granulysins
Fas-mediated apoptosis:
via cell surface interaction between Tc and the infected cell
When a TC is activated it starts to express the surface protein FAS ligand (FasL)(Apo1L)(CD95L)
The surface protein FAS ligand can bind to Fas (Apo1)(CD95) molecules expressed on the target cell.
those molecules always on the Th cells
so this interaction are most to remove the unwanted T lymphocytes
downstream
Engagement of Fas with FasL allows for recruitment of the death-induced signaling complex (DISC). The Fas-associated death domain (FADD) translocates with the DISC, allowing recruitment of procaspases 8 and 10. These caspases then activate the effector caspases 3, 6, and 7, leading to cleavage of death substrates such as lamin A, lamin B1, lamin B2, PARP (poly ADP ribose polymerase), and DNAPK (DNA-activated protein kinase). The final result is apoptosis of the cell that expressed Fas.
Upregulate FasL via Fas-FasL
other IFN released
invovled in pathogenesis of HBV, CTL can release Cytokines to against HBV
MHCI
Cells expressing MHCI
all the potential host cells(the cells that can be infected by intracellular pathogens)
most nucleoated cells
called antigen presenting cells (APC) when they stimulating PCR on CTL
antigen processing
1. peptide fragmentation
NK and CTL are complementing eachother?
recognizing alter-self cell
T cells are unable to recognize pathogens in the absence of surface antigens
NK cells can detect the abnormal cells even in the absence of surface adhesion molecules and antigenic peptides(mostly down regulation of MHCI)
but there is no imflammation, there is no NK cells, so sometimes the tumor can develop...
CD8 cells can consequently only act on tumor cells in response to NK initiated cytokine production (adaptive immune response)
All of them presents only in "vertebrates"