导图社区 Coenzymes(psuedoscince naming)
Coenzymes(psuedoscince naming)
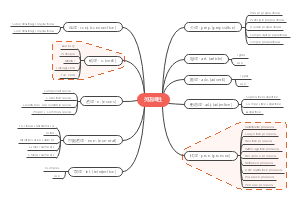
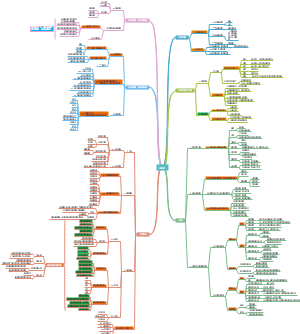
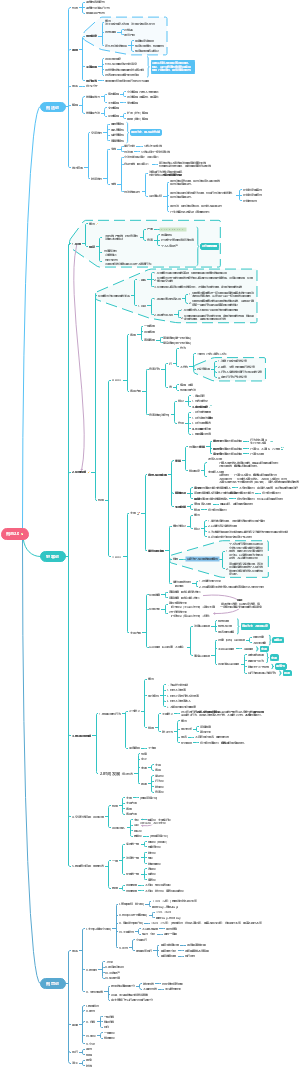
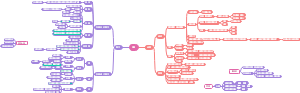
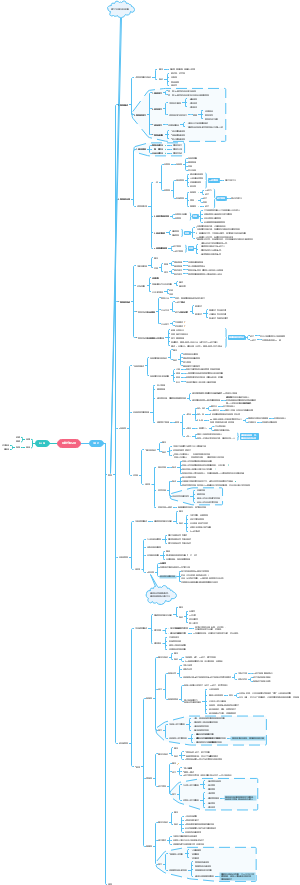
这是一篇关于Coenzymes(psuedoscince naming)的思维导图,主要内容有coreactant phospate carriers、coreactant hydride ion e- carriers、prosthetic e- transfer groups、defination等。
编辑于2022-06-11 15:52:19- Coenzymes
- prosthetic…
- coreactan…
- ion e- car…