导图社区 Chaperons
- 19
- 1
- 1
- 举报
Chaperons
这是一篇关于Chaperons的思维导图,主要内容有Structures、Functions、lnduction、Mechanisms等。
编辑于2022-06-11 15:54:18- Chaperons
- Hsp70
- DNAK
- DNAJ(co-…
- 相似推荐
- 大纲
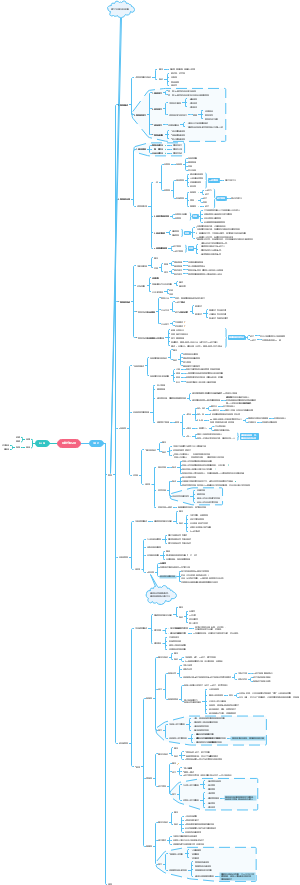
Chaperons
Structures
Globular
Hydrophobic region inside
some hydrophilic amino acids outside
peptide binding sequence
DNAK
short 7 - 9 residues
hydrophobic segments with basic residues at ends
extended conformation.
No acidic residues are tolerated.
Bip
large aromatics; trp, phe, tyr.
won’t tolerate basic residues
Hsp70
primary:ATP binding + ATPase domain44kDa---linker----protein binding domain13kDa----lid 10kDa
ATP binding closes the lid and holds the binding peptide
high affinity for protein binding in ADP binding form
low affinity for protein binding in ATP binding form
Functions
Refolding
most proteins have chaperones
prevent many damages, can induced as therapeutic agent
prevent aggregation
Signal for protein targeting
targets Intracellular membrane transporter
Also Degredation targeting
e.x.Bip for ER targeting
Immune response
Intracellular
Cell surface antigen
autoimmune responce
can be used as vaccines
recognize by specific antigen presenting cells: macrophages, dendritic cells
first it bonds to sAPC surface CD91
sAPC ingest the antigen and present it on its surface MHCII
sAPC migrates to lymph nodes and activates Th cells
Th cells undergo clonal expansion, and activates other immune cells with same targeting specificity
Different types of cancer cells have cirtain profile of chaperon expression
Extracellular
intracellular antigens
possible binding targets
Protein folding during synthesis
Cytoskeleton
Organelles
Disruption of protein translation
Enzyme complexes in energy metabolism
DNA synthesis and transcription
Cell membranes/membrane proteins
Induction
Stress condation
Hot/Cold
Chemical
low Oxygen level
oxygen free radicals
Aminoacid analogous?
toxic metals
so some chaperons can be used as pollution detector..
Cellular activities
energy metabolism inhibition
Cell cycle
growth factors
oncogenes
differenciation
ex. cirtain chaperon levels different in brain cells which cause short-term/long-term memories
Physiological
Fever inflammation
Neurohormonal stress
Hypertrophy
Oxidant injury
Ischaemia
Anti-neoplastics
Viral/bacterial infection
Tissue injury, repair
Ageing
normal condation(cognate)
also express HSC chaperones
Regulation of HSP70
By heat shock transcription factor HSF
Trimer is activated form, but the dissociation of trimer is catalyst by Hsp70 itself
feed back inhibition
Mechanisms
DNAK + DNAJ(co-chaperones)
DNAJ binds misfold/partially misfold protein
mP-DNAJ binds DNAK-ATP
mp-DNAJ-DNAK-ATP hydrolysis it self ATP into mp-DNAJ-DNAK-ADP(tighter structure)
The mp get a chance to become nP(Ntural protein) here, or pmP(partially misfold proteins)
most time may fail here
however the mp-DNAJ-DNAK-ADP can prevent mPs aggregation
Do not need ATP renew
GRPE dissociates ADP and DNAJ from the complex
ATP binds to mp-DNAJ into mp-DNAK-ATP
mp dissociate from DNAK-ATP
3 possible out comes
if pmP it contaneus go through the cycles
need ATP renew
if nP it finish
if still mp it may targeted to degredation
Hsp 70
signal for prontien targeting
target synthesizing protein
1. Hsp70 binds to ribosome elogationg peptide
2. BiP then binds to peptide, and target peptide it to ER/mitochondria
target it on clathrin coat of endosome vesicles
as a protein signal
target it to lysosome degredation
restore/protection
cytoskeleton
energy metabolism enzymes
in nucleus protect nucleus proteins
target very damaged proteins to proteosome degredation
Structure
primary:ATP binding + ATPase domain44kDa---linker----protein binding domain13kDa----lid 10kDa
high affinity for protein binding in ADP binding form
low affinity for protein binding in ATP binding form
hsp70-ATP associates with a protein's short hydrophobic segments, ATP hydrolysis after binding, and hsp70-ADP shields the protein from aggregation
just like DNAK and with out assist of DNAJ
it binds directly to the misfolded protein, and hydrolysis it self ATP
Further speeding ATP hydrolysis are the so-called J-domain cochaperones: primarily Hsp40 in eukaryotes,
protein conformation
NATURE
nature conformationed protein has function
DENATURE
AGGREGATES
one of denatured conformation, proteins aggregates and form clumps, this disfuction cells and cause issue/ diseases
solution
1. degredation by proteosome
2. chaperone
Factors cause denaturation
temperature
ΔG=ΔH-TΔS
Mamaline cells should survive at least under 44℃
other chemical enviroments
Oxygen?
PH
Evidences
SDS pages
during heat stress, cell most only synthesis heat shock proteins
Chromosomal structure
Heatshocks activates transcription around cirtain region of chromosomes
Biochemical study
DNAK increase RNApol activity against heat
DNAK can restore RNApol activity after they've been denatured
We injects Hsp70 to cells can increase cells heat tolerance
Genetics study
animal
over express of HSP in drasophila increase individual's heat tolerance
bacteria
DNAK knockout decrease E.coli heat tolerance
also DNAK over express increase various stress tolerance
Classes
Eukaryotic
hsp 100
numbers mean molecular weight in kDa
targetting Protein disaggregation, thermotolerance
hsp 90
Regulates cell signaling, stabalize misfold proteins
hsp 70
bacteria homologous: DNAK
transport proteins on membrane, proteins folding
family
hsp72
nucleus, cytosol
not cognate
massive increase when heat shock
hsp70+73
in nucleus, cytosol
cognate
no increase when HS
Bip(Binding immunoglobin proteins)
in ER
cognate
no increase when HS, increase when starvation and other stresses
protein localized to the endoplasmic reticulum. It is involved in protein folding there
specific hsp70
only for mitochondria, chloroplast, and lysosome
hsp 60
cytoplasmic proteins folding
hsp 40
co-chaperons for hsp70, protein folding
Prokaryotic homologous DNA J
other smaller hsps
most stabalize misfold proteins
some can be structural proteins in eye lens