导图社区 有机化学烷烃-链烷烃导图(中英)
- 4
- 举报
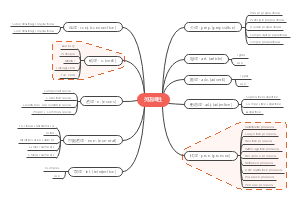
有机化学烷烃-链烷烃导图(中英)
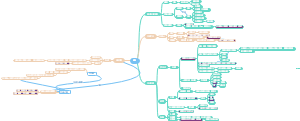
这是一篇关于有机化学烷烃-链烷烃导图(中英)的思维导图,链烷烃是一类有机化合物,其分子中的碳原子以单键相连形成链状结构,其余的价键都与氢原子结合。这种结构使得链烷烃成为饱和烃的一种,即分子中的碳原子已经达到了与氢原子结合的最大限度。
编辑于2024-09-20 13:36:20- 有机化学
- 烷烃
- 有机化合物
- 相似推荐
- 大纲
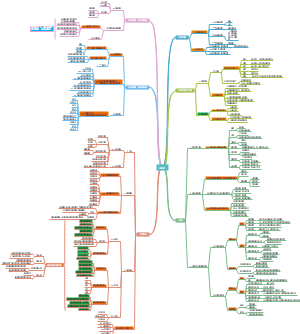
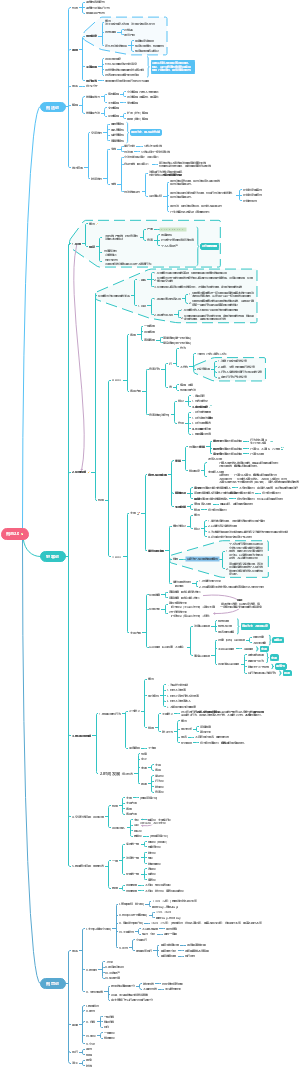
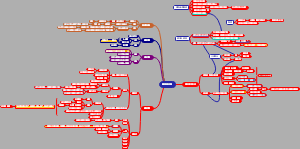
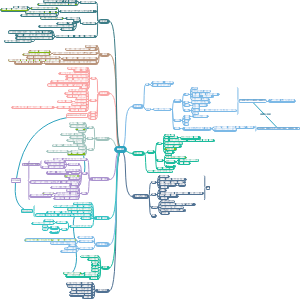
Ch3/4 Organic Compounds
Cycloalkanes
Naming
alicyclic(脂环)/CnH2n
Steps
① Find the parent
number in the largest substituent
cycloalkane/alkane
② Number the substituents
③ Write the name
Structure & stability
Cis-trans isomerism
less flexible
lesser conformational freedom
Stability: Ring Strain
Torsional strain (扭转张力)
the strain due to eclipsing of bonds between neighboring atoms
Steric strain(空间张力,非键张力)
the strain due to expansion or compression of bond angles
Angle strain(角张力)
forced to deviate from the ideal 109.5°tetrahedral value
the strain due to expansion or compression of bond angles
Heat of Combustion (燃烧热)
heat of 1 mol of compound burns completely with oxygen to form CO2 and H2O
Conformations
Cycloalkanes
Cyclopropane
Most strained - angle strain by C–C–C of 60°
torsional strain
Has bent bonds
C–H bonds are eclipsed
Cyclobutane
Has less angle strain
More torsional strain
one carbon atom is 25° above the plane
Cyclopentane
No angle strain
Large torsional strain
Cyclohexane
Conformations
Chair conformation
Strain-free
Neither angle strain nor torsional strain
Boat cyclohexane
No angle strain
Large number of eclipsing interactions
Twist-boat conformation
more stable than pure boat conformation
Nearly free of angle strain
Axial & Equatorial bonds/positions
Axial bond(直立键)(a键)
perpendicular to the ring
Equatorial bond(平伏键)(e键)
near the plane of the ring
Conformational Mobility
Ring-flip: Interconversion of chair conformations, resulting in the exchange of axial and equatorial positions
Monosubstituted
Substituent - more stable in equatorial than axial position
Disubstituted
Cis isomer
Both methyl groups on the same face of the ring
Compound exists in two chair conformations
Trans isomer
Methyl groups are on opposite faces of the ring
No 1,3-diaxial interactions
Ring-flipped conformation has both methyl groups axial
Exist almost exclusively in diequatorial conformation
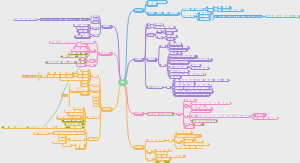
Alkanes
Naming
IUPAC system
① Find parent hydrocarbon chain
longest continuous chain
with the larger number of branch points
② Number the atoms
nearer to the second branch point
③ Identify and number substituents
④ Write the name as a single word
Tips
hyphens(-) → prefixes and commas(,)→ numbers
cite different side chains in alphabetical order
cite identical side chains after di-,tri-, tetra...
not for alphabetizing!
only prefix "iso-"used for alphabetizing
to name a branched substituent as compound
Structure & Stability
Alkane & Alkane Isomers
Alkanes:
Def: Compounds with C–C single bonds & C–H bonds
Saturated hydrocarbons
Also called aliphatic compounds
an alkane with more than three carbons can give more than one structure
Alkane Isomers
Straight-chain alkanes
Branched-chain alkanes
Alkanes with one or more carbon atoms connected to 3 or 4 C
Isomers
Constitutional Isomers
differs in how their atoms are arranged in chains
Condensed Structure of Alkanes
Condensed structure does not show bonds but lists atoms
Alkyl Groups
Prefixes are used to represent the number of other carbon atoms
1°= primary carbon
2° = secondary carbon
3° = tertiary carbon
4°= quaternary carbon
not stable compounds
named by replacing –ane with –yl
R → neralized organic group
Properties
Called paraffins
Low affinity
Burn in flame, producing CO2, H2O, and heat
Physical Properties
Solubility
Hydrophobic
Boiling points & melting points increase as size of alkane increases
Dispersion forces increase as molecule size increases, resulting in higher melting and boiling points
Branched alkanes
Lower b.p. with increased branching
Higher m.p. with increased branching
Conformations
Ethane
Stereochemistry(立体化学)
σ bonds
Bond rotation
conformer presentation
Sawhorse representation
Wedge-and-dash(楔形)
Newman projection(纽曼投影)
Torsional Strain(扭转张力)
Staggered conformation: Most stable(错位)
Eclipsed conformation: Least stable(重叠)
Other alkanes
Propane - eclipsed conformer -3 interactions
Butane
Anti conformation (对位交叉)
two larger groups stagger 180°
Gauche conformation (邻位交叉)
two larger groups stagger 60°
Functional Groups
Def: Collection of atoms at a site that have a characteristic behavior in all molecules where they occur
Multiple Carbon-Carbon Bonds
Alkenes -- double bond
Alkynes -- triple bond
Arene -- alternating double & single C-C bonds
Singly bonded to an electronegative atom
a C– O double bond (Carbonyl Groups)