导图社区 生物学思维导图
- 132
- 1
- 1
- 举报
生物学思维导图
生物学第三章讲述了水分子中的极性共价键是氢键、水的四个涌现特征、酸性合碱性条件影响生物体、海洋酸化等内容。
编辑于2021-08-27 12:02:37- 相似推荐
- 大纲
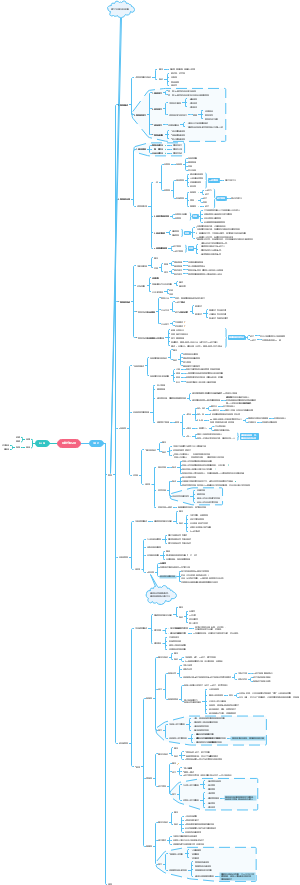
chapter 3
polar covalent bond in water molecules is hydrogen bond
The partially positive hydrogen of one molecule is attracted to the partially negative oxygen of a nearby molecule. water is in its liquid form, its hydrogen bonds are very fragile, each only about 1/20 as strong as a covalent bond.
Four emergent properties of water
Cohesion of Water Molecules
Collectively, the hydrogen bonds hold the substance together, a phenomenon called cohesion. This will cause high surface tension.
Adhesion, the clinging of one substance to another
moderation of temperature
The ability of water to stabilize temperature stems from its relatively high specific heat. The specific heat of a substance is defined as the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1°C
Evaporative cooling
Heat of vaporization is the quantity of heat a liquid must absorb for 1 g of it to be converted from the liquid to the gaseous state
Floating of Ice on Liquid Water
Water is one of the few substances that are less dense as a solid than as a liquid
reason: When water is frozen into a lattice, the hydrogen bonds between the water molecules are frozen to a fixed length, leaving space between the water molecules. When the water returns to its liquid state, the heat absorbed by the water breaks the hydrogen bonds, bringing the water molecules closer together
Water: The Solvent of Life
solution: A liquid that is a completely homogeneous mixture of two or more substances is called a solution
solvent: The dissolving agent of a solution is the solvent
solute: the substance that is dissolved is the solute
versatile solvent: Water molecules surround the individual sodium and chloride ions, separating and shielding them from one another. The sphere of water molecules around each dissolved ion is called a hydration shell
Hydrophilic and Hydrophobic Substances
Any substance that has an affinity for water is said to be hydrophilic
Substances that are nonionic and nonpolar (or otherwise cannot form hydrogen bonds) are hydrophobic
Solute Concentration in Aqueous Solutions
Molarity—the number of moles of solute per liter of solution—is the unit of concentration most often used by biologists for aqueous solutions.
Acidic and basic conditions affect living organisms
Acids and Bases
Acids
When acids dissolve in water, they donate additional H+ to the solution. For example, when hydrochloric acid (HCl) is added to water, hydrogen ions dissociate from chloride ions:
Bases
A substance that reduces the hydrogen ion concentration of a solution is called a base. for instance when the unshared electron pair in nitrogen’s valence shell attracts a hydrogen ion from the solution, resulting in an ammonium ion (NH4+):
PH scale:
Buffer: A buffer is a substance that minimizes changes in the concentrations of H+ and OH- in a solution. Most buffer solutions contain a weak acid and its corresponding base, which combine reversibly with hydrogen ions.
Acidification: Ocean Acidification
When CO2 dissolves in seawater, it reacts with water to form carbonic acid, which lowers ocean pH. This process, known as ocean acidification, alters the delicate balance of conditions for life in the oceans.