导图社区 基础化学-化学热力学
- 136
- 2
- 0
- 举报
基础化学-化学热力学
这是一篇关于基础化学-化学热力学的思维导图,主要内容有First Law of Thermodynamics、Second Law of Thermodynamics、Third Law of Thermodynamics等。
编辑于2022-09-18 12:03:03 陕西- 热力学
- 相似推荐
- 大纲
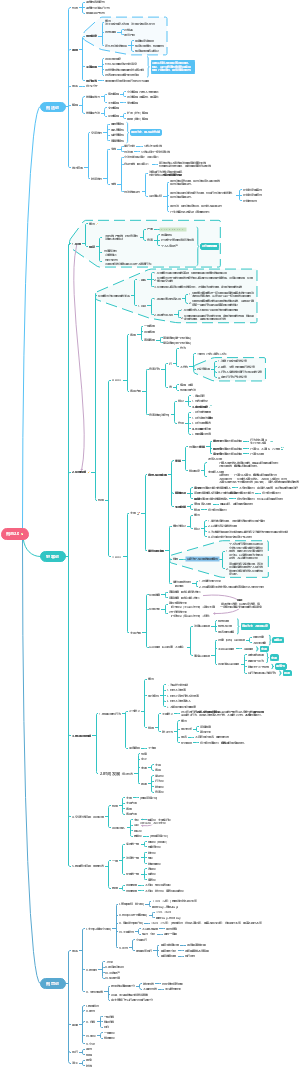
Fundamental Chemistry Chapter one Chemi-hermodynamics
Three Laws of Thermodynamics
First Law of Thermodynamics
Conservation of energy in an isolated system 能量守恒和转化定律 Mayer(1842) Joule、Carnot
Enthalpy
isovolumic heat isobaric heat
H≡U+pV
系统从环境吸热,DH>0 Qv=DU,Qp=DH Qp=Qv+Dn(RT)
体积变化极小时,Qp»Qv
When the isobaric process only does volume work, the heat absorbed or released by the system is all used to change the enthalpy of the system. 等压过程只做体积功时系统吸收或放出的热,全部用于改变系统的焓值。
Thermal effects of chemical reactions(heat effect)
摩尔反应热效应DrHmq(T) (298K,100kPa)
Hess's law
A chemical reaction, whether completed in one step or in several steps, always has the same thermal effect. 一个化学反应,不论是一步完成还是分几步完成,其热效应总是相同的。
标准摩尔生成焓(最稳定单质间)
标准摩尔燃烧焓(最稳定燃烧产物)
direction of chemical reaction
spontaneous process
方向的单一性
具有做功的能力
有一定限度
entropy
system chaos
At absolute zero degrees, a perfect crystal of any pure substance has zero entropy.
DS=ST-S0=ST
principle of entropy increase: In an isolated system, the spontaneous process always proceeds in the direction of entropy increase, that is, order → disorder.
DS总>0,反应自发进行 DS总=0,反应平衡 DS总<0,反应逆向自发进行
S:热温商 S=klnW
Gibbs function
G=H-TS
D G>0,反应逆向自发进行 DG=0,反应平衡 DG<0,反应自发进行
DG=DH-TDS
Gibbs-Helmholez公式
ΔU=Q+W
Second Law of Thermodynamics
The irreversibility of the heat conduction process 热传导过程的不可逆性 Clausius(1850)、Kelvin
Third Law of Thermodynamics
Absolute zero is unattainable but infinitely close 绝对零度不可达到但可以无限接近 Nernst(1912) Planck、Gibbs
Zeroth Law of Thermodynamics
Basic Thermodynamic Terminology
system&surroundings
system
open system (Exchange matter and energy with the environment)
closed system (Only exchanges energy with the environment, not matter) (Most important)
isolated system (No exchange of energy, no exchange of matter with the environment)
surroundings
state&state fuctions
state
Comprehensive performance of all macroscopic properties of the system A series of macroscopic physical quantities
T,p,V,n,ρ,U,H,S
initial state~final state
state fuctions
Various thermodynamic macroscopic properties
Feature
The state of the system is constant, and the state function has a certain value 系统状态一定,状态函数就有一定的值
When the state of the system changes, the amount of change in its state function only depends on the initial and final states of the system. regardless of the path of change 系统状态发生变化时,其状态函数的变化量只取决于系统的始态和终态, 而与变化的途径无关
Once the system returns to its original state, the state function returns to its original value 系统一旦恢复到原来状态,状态函数即恢复原值
Property
extensive property (additive)
intensive property (non-additive)
Conditions for Thermodynamic Equilibrium
thermal equilibrium(热平衡)
mechanical equilibrium(力平衡)
phase equilibrium(相平衡)
chemical equilibrium(化学平衡)
process&path
process
Any change in system state
isothermal process(等温) isobaric process(等压) isovolumetric process(等容) adiabatic process(绝热) cyclic process(循环)
IDEAL PROCESS:reversible process (Quasi-static process, continuous change without sudden change)(Does not consider external work)
path
The specific steps of the system to complete a process
Different paths do not affect the amount of change in the state function
heat&work
heat
Q
The system absorbs (releases) heat to the environment, and Q is a positive (negative) value,Q>(<)0
work
W
The system does work on the environment, and W is a negative value,W<0
Volume work (wasted work):W体 Non-volume work (useful work):W'
W体= -F ·l = -(p外· A) ·l= -p外 ΔV W体= – p外ΔV
Work is not a state fuction
ideal gas isothermal expansion
one step expansion
multi-step expansion
Infinite step expansion (reversible expansion)
1.Unlimited time required 2.Each step of the reversible process is infinitely close to the equilibrium state 3.A reversible process is actually an impossible ideal process
Thermodynamic energy (internal energy)
E=T(体系动能)+V(体系势能)+U(内能) In the chemical reaction system, T=V=0, then E=U.
chapter1~10
Chapter1~3
Chemical Thermodynamics & Chemical Kinetics
First Law of Thermodynamics
Thermal effects of chemical reactions
The direction and limits of chemical reactions
Chapter4&6
Dilute solution & solution system
Chapter5,7,10
Four reaction balances
Chapter8~9
Principles of Inorganic Chemistry