导图社区 Cambridge IGCS Chemistry Coursebook 2023 Chapter 9 知识点整理
- 10
- 0
- 0
- 举报
Cambridge IGCS Chemistry Coursebook 2023 Chapter 9 知识点整理
这是一篇关于Reversible reactions and equil的思维导图,主要内容包括:Reversible reactions,Fertilisers,Haber process and Contact proces。
编辑于2024-12-14 12:45:41- 知识点整理
- 英文版
- IGCSE化学
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
Cambridge IGCS Chemistry Coursebook 2023 Chapter 9 知识点整理
社区模板帮助中心,点此进入>>
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
- 相似推荐
- 大纲
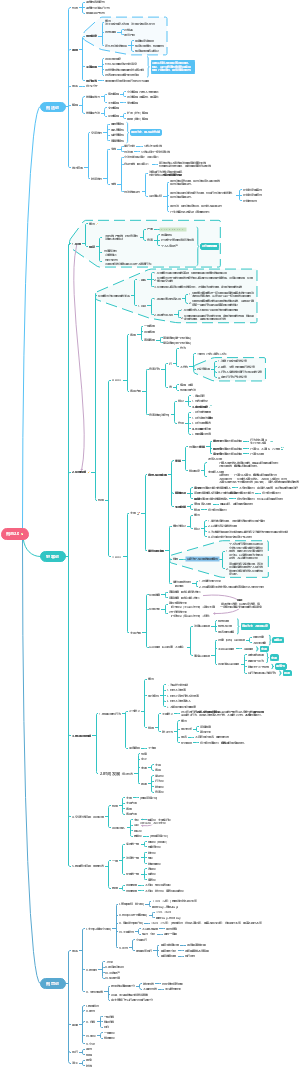
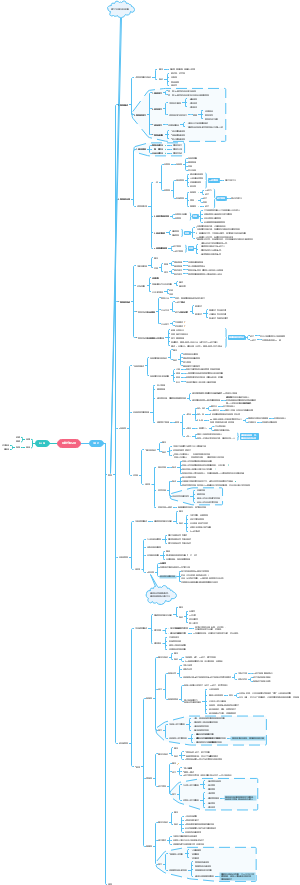
Reversible reactions and equilibrium
Haber process and Contact proces
Haber process
Fertiliser: a substance added to the soil to replace essential elements lost when crops are harvested, which enables crops to grow faster and increases the yield.
Conditions for the Haber process
compromise temperature: a temperature that gives sufficient product and a reasonable and economic rate of reaction
N2(g) + 3H2(g) ⇌ 2NH3(g)
Pressure
4 moles ⇌ 2 moles
Temperature
exothermic ->
endothermic <-
Catalyst
• N2 and H2 are mixed in a ratio of 1:3 • an optimum (or compromise) temperature of 450 °C • a pressure of 20 000 kPa (200 atmospheres) • a catalyst of finely divided iron.
Industrial plant for the Haber process
CH4(g) + H2o(g) → CO(g) + 3H2(g)
CO(g) + H2O(g) → CO(g) + H2(g)
C2H6(g) → C2H4(g) + H2(g)
Contact process
Conditions for the Contact process
2SO2(g) + O2(g) ⇌ 2SO3(g)
exothermic (ΔH = -197 kJ / mol )
• a compromise (optimum) temperature of about 450°C • a catalyst of vanadium(V) oxide (V2O5) • an operating pressure of 200 kPa.
Industrial plant for the Contact process
Desulfurisation: an industrial process for removing contaminating sulfur from fossil fuels such as petrol (gasoline) or diesel
S(s) + O2(g) → SO2(g)
2ZnS(s) + 3O2(g) → 2ZnO(s) + 2SO2(g)
2SO2(g) + O2(g) → 2SO3(g)
SO3 + H2O → H2SO4
SO3 + H2 SO4 → H2S2O7
Fertilisers
NH3(aq) + HNO3(aq) → NH4NO3(aq)
• phosphorus (P), especially important for healthy roots • potassium (K), which is important for the production of flowers and fruit.
• Straight N fertilisers are solid nitrogen-containing fertilisers sold in pellet form, for example, ammonium nitrate (NH4NO3), ammonium sulfate (NH4)2SO4) and urea (CO(NH2)2)
• NPK compound fertilisers are mixtures that supply the three most essential elements lost from the soil by extensive use: nitrogen (N), phosphorus (P) and potassium (K). They are usually a mixture of ammonium nitrate, ammonium phosphate and potassium chloride, in different proportions to suit different conditions. The numbers on a bag of NPK fertiliser correspond to the percentage of that nutrient in the make-up of the fertiliser, e.g. 21:8:11 indicates 21% nitrogen, 8% phosphorus and 11% nitrogen, with the remaining 60% being filler ingredients that help disperse the chemicals. Nitrogen is sometimes omitted from these fertilisers because it washes into streams and rivers causing algal growth.
Compound fertiliser: a fertiliser such as an NPK fertiliser or nitrochalk that contains more than one compound to provide elements to the soil
NPK fertiliser: fertilisers to provide the elements nitrogen, phosphorus and potassium for improved plant growth
Reversible reactions
Reversible hydration of salts
CuSO4 · 5H20(s) ⇌ CuSO4(s) + 5H2O(g)
light blue crystals ⇌ white powder
CoCL2 · 6H20 ⇌ CoCl2 + 6H2O
pink ⇌ blue
Chemical test for the presence of water
Chemical equilibria
Closed system: a system where none of the reactants or products can escape the reaction mixture or the container where the reaction is taking place
Dynamic (chemical) equilibrium: two chemical reactions, one the reverse of the other, taking place at the same time, where the concentrations of the reactants and products remain constant because the rate at which the forward reaction occurs is the same as that of the reverse reaction
• It is dynamic: reactants are continuously being changed to products and products are continuously being changed back to reactants. • The forward and reverse (backward) reactions occur at the same rate. • The concentrations of reactants and products (the position of equilibrium) remain constant. • It requires a closed system.
Chemical equilibria and reaction conditions
Contact process: the industrial manufacture of sulfuric acid using the raw materials sulfur and air
Temperature: Increasing the temperature makes the reaction move in the direction that takes in heat (the endothermic direction). Decreasing the temperature makes the reaction move in the direction that gives out heat (the exothermic direction).
Pressure: This only affects reactions involving gases. Increasing the pressure shifts the equilibrium in the direction that produces fewer gas molecules. Decreasing the pressure shifts the equilibrium in the direction that produces more gas molecules.
Concentration: Increasing the concentration of one substance in the mixture makes the equilibrium move in the direction that produces less of that substance. Decreasing the concentration of one substance in the mixture makes the equilibrium move in the direction that produces more of that substance.
Catalyst: Using a catalyst does not affect the position of equilibrium, but the reaction reaches equilibrium faster.
Reversible reaction: a chemical reaction that can go either forwards or backwards, depending on the conditions
hydrated salts: salts whose crystals contain combined water (water of crystallisation) as part of the structure
Anhydrous: an adjective to describe a substance without water combined with it