导图社区 Cambridge IGCS Chemistry Coursebook 2023 Chapter 12 知识点整理
- 19
- 0
- 0
- 举报
Cambridge IGCS Chemistry Coursebook 2023 Chapter 12 知识点整理
这是一篇关于Preparation of salts的思维导图,主要内容包括:Preparation of salts,The importance of salts。
编辑于2024-12-14 22:49:52- 英文版
- IGCSE化学
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
Cambridge IGCS Chemistry Coursebook 2023 Chapter 12 知识点整理
社区模板帮助中心,点此进入>>
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 21 知识点整理
This is a mind map about Experimental design and separa,Main content: Chromatography,Separation and purification,Experimental design。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 20 知识点整理
This is a mind map about Petrochemicals and polymers,Main content: Plastics,Polymers,Petroleum and its products。
- Cambridge IGCS Chemistry Coursebook 2023 Chapter 19 知识点整理
This is a mind map about Reactions of organic compounds,Main content: Carboxylic acids and esters,Chemistry of ethanol,Characteristic reactions of different homologous series。
- 相似推荐
- 大纲
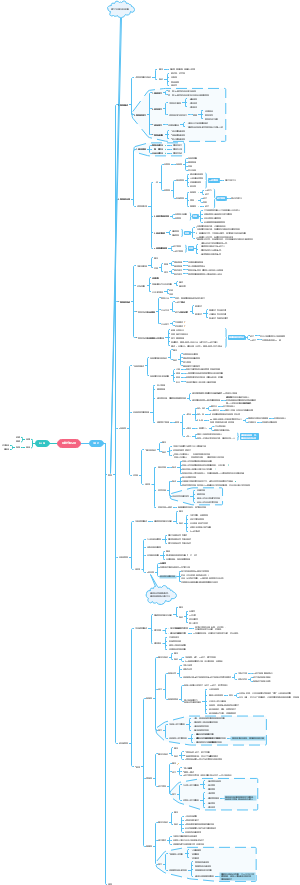
Preparation of salts
The importance of salts
salts: ionic compounds made by the neutralisation of an acid with a base (or alkali), e.g. copper(II) sulfate and potassium nitrate
Solubility of salts
soluble: a solute that dissolves in a particular solvent
insoluble: a substance that does not dissolve in a particular solvent
hydrated substance: a substance that is chemically combined with water; hydrated salts are an important group of such substances
Anhydrous: an adjective to describe a substance without water combined with it
water of crystallisation: water included in the structure of certain salts as they crystallise, e.g. copper(II) sulfate pentahydrate (CuSO · 5H2O) contains five molecules of water of crystallisation per molecule of copper(II) sulfate
Preparation of salts
Preparing soluble salts
Choosing a method of salt preparation
Method A - acid plus solid metal, base or carbonate
Stage 1: An excess of the solid is added to the acid and allowed to react. Using an excess of the solid makes sure that all the acid is used up. If it is not used up at this stage, the acid would become more concentrated when the water is evaporated later (stage 3).
Stage 2: The excess solid is filtered out.
Stage 3: The filtrate is gently evaporated to concentrate the salt solution. This can be done on a heated water-bath or sand tray.
Stage 4: When crystals can be seen forming (crystallisation point), heating is stopped and the solution is left to crystallise.
Stage 5: The concentrated solution is cooled to let the crystals form. The crystals are filtered off and washed with a little distilled water. Then the crystals are dried carefully between filter papers.
Method B - acid plus alkali by titration
titration: a method of quantitative analysis using solutions: one solution is slowly added to a known volume of another solution using a burette until an end-point is reached
burette: a piece of glass apparatus used for delivering a variable volume of liquid accurately
volumetric pipete: a pipette used to measure out a volume of solution accurately
end-point: the point in a titration when the indicator just changes colour showing that the reaction is complete
Stage 1: The acid solution is poured into a burette. The burette is used to accurately measure the volume of solution added. A known volume of alkali solution is placed in a conical flask using a volumetric pipette. The pipette delivers a fixed volume accurately. A few drops of an indicator (e.g. thymolphthalein or methyl orange) are added to the flask.
Stage 2: The acid solution is run into the flask a few drops at a time from the burette until the indicator just changes colour (Figure 12.12). The conical flask must also be swirled after each portion of acid to ensure everything is mixed and the reaction is complete. Having found the end-point for the reaction, the volume of acid (titre volume) added is noted. The experiment is then repeated without using the indicator. The same known volume of alkali is used in the flask. The same volume of acid as noted in the first part is then run into the flask. Alternatively, activated charcoal can be added to remove the coloured indicator. The charcoal can then be filtered off.
Stage 3: The salt solution is evaporated and cooled to form crystals as described in method A.
Preparing insoluble salts by precipitation
precipitation: the sudden formation of a solid when either two solutions are mixed or a gas is bubbled into a solution
ionic equation: the simplified equation for a reaction involving ionic substances: only those ions which actually take part in the reaction are shown
BaCl(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
Ba 2+ (aq) + SO4 2-(aq) → BaSO4(s)
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Ag+(aq) + Cl-(aq) → AgCl(s)
Pb 2+(aq) + 2I- (aq) → PbI2(s)